Figure 2. Pak regulates actin polymerization but not MLC phosphorylation in tracheal smooth muscle tissues during contractile stimulation .
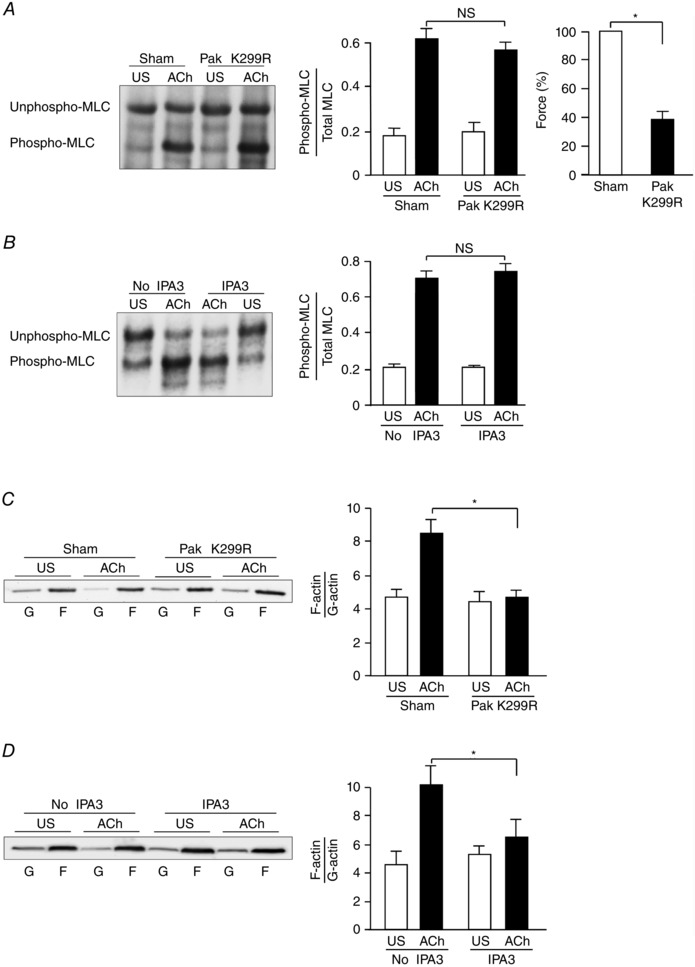
A, representative immunoblot obtained from urea gel electrophoresis of extracts of four muscle tissues treated with Pak K299R or sham‐treated and then stimulated with ACh or unstimulated (US) (left). Unphosphorylated and phosphorylated smooth muscle 20 kDa MLCs were quantified as the ratio of phosphorylated MLCs to total MLCs in each sample (middle). Expression of the Pak K299R did not significantly affect MLC phosphorylation in response to ACh, but it significantly inhibited ACh‐induced tension development of smooth muscle tissues (n = 8) (right). The absolute value of the mean force of the sham‐treated tissues was 88.2 ± 5.0 mN mm−2. B, representative immunoblot obtained from urea gel electrophoresis of extracts of four muscle tissues with or without IPA3 treatment and stimulated with ACh or unstimulated (US) (left). IPA3 did not significantly affect MLC phosphorylation in response to ACh (n = 6) (right). C, immunoblot of soluble G‐actin (globular) and insoluble F‐actin (filamentous) in fractions from extracts of unstimulated (US) or ACh‐stimulated muscle tissues treated with Pak K299R or sham‐treated. Ratios of F‐actin to G‐actin were determined by quantifying F and G actin in extracts from each muscle strip (left). Inhibition of Pak activation with Pak K299R prevented the increase in F‐actin/G‐actin ratio in response to ACh stimulation (n = 6) (right). D, inhibition of Pak activation with IPA3 prevented the increase in F‐actin/G‐actin ratio in response to ACh stimulation (n = 6). Values are means ± SEM. *Significant difference between treatments, P < 0.05. NS, not significantly different.
