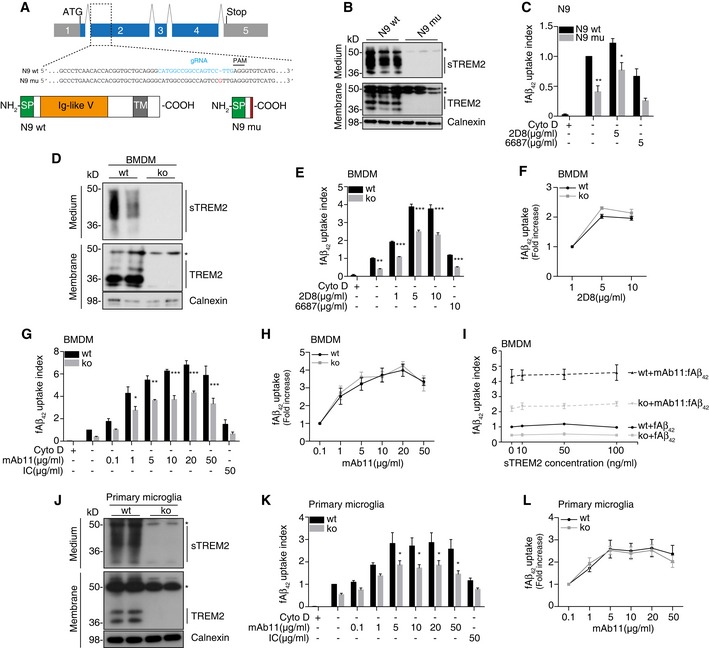
Schematic of the mouse
Trem2 locus and the TREM2 protein. Sequence alignment of wild‐type N9 (N9 wt) and TREM2 mutant N9 (N9 mu) surrounding the gRNA target site. The gRNA sequence is in cyan, and protospacer‐adjacent motif (PAM) is marked with a line. The single nucleotide insertion is labeled in red. Schematic representation of wild‐type TREM2 (
NP_112544.1) and CRISPR/Cas9‐modified TREM2 (N9 mu). TM, transmembrane domain; SP, signal peptide.
Western blot analysis of lysates and media from wt and mutant N9 cells (N9 wt /mu) using the antibody anti‐murine TREM2 (clone 5F4), which is raised against the murine TREM2 extracellular domain. sTREM2, soluble TREM2. *indicate unspecific bands. Calnexin was used as a loading control.
Phagocytosis of 1 μM HiLyte™ Fluor 488 Aβ1‐42 (fAβ42) by N9 wt and N9 mu in the presence or absence of antibody 2D8 or the non‐binding antibody 6687. Cytochalasin D (CytoD, 10 mM) was used as control to verify phagocytic uptake. (n = 4, ± SEM; two‐way ANOVA, interaction P = 0.61, genotype P < 0.0001, treatment P = 0.0001; post hoc tests wt vs. mu for the following conditions: fAβ42
P = 0.0043, fAβ42‐2D8 P = 0.0436).
Western blot of BMDM derived from wt and Trem2 knockout (ko) animals using antibody 5F4. *indicate unspecific bands.
Phagocytosis of fAβ42 by BMDM from wt and Trem2 ko animals in the presence or absence of 2D8, or the non‐binding control antibody 6687. (n = 3, ± SEM; two‐way ANOVA, interaction P = 0.0005, genotype P < 0.0001, treatment P < 0.0001; post hoc tests wt vs. ko for the following conditions: fAβ42
P = 0.0021, fAβ42‐2D8 1 μg/ml P < 0.0001, fAβ42‐2D8 5 μg/ml P < 0.0001, fAβ42‐2D8 10 μg/ml P < 0.0001, fAβ42/6687 10 μg/ml P = 0.0007).
Quantification of relative fAβ42 uptake to lowest antibody concentration used (n = 3, ± SEM).
Phagocytosis of fAβ42 by BMDM from wt and Trem2 ko animals in the presence or absence of mAb11, or an isotype control antibody (IC). (n = 4, ± SEM; two‐way ANOVA, interaction P = 0.0223, genotype P < 0.0001, treatment P < 0.0001; post hoc tests wt vs. ko for the following conditions: fAβ42‐mAb11 1 μg/ml P = 0.0391, fAβ42‐mAb11 5 μg/ml P = 0.0069, fAβ42‐mAb11 10 μg/ml P < 0.0001, fAβ42‐mAb11 20 μg/ml P = 0.0001, fAβ42‐mAb11 50 μg/ml P < 0.0001).
Quantification of relative fAβ42 uptake to lowest antibody concentration used (n = 4, ± SEM).
Recombinant mouse sTREM2 does not rescue fAβ42 uptake in Trem2‐deficient BMDM. Increasing amounts of sTREM2 were added to the media of wt or Trem2 ko BMDM in the presence or absence of mAb11 (10 μg/ml) (n = 4, ± SEM).
Western blot of primary microglia from wt or Trem2 ko animals using antibody 5F4. *indicate unspecific bands.
Phagocytosis of fAβ42 by primary microglia from wt and Trem2 ko animals in the presence or absence of mAb11, or an isotype control antibody (IC). (n = 5, ± SEM; two‐way ANOVA, interaction P = 0.4797, genotype P < 0.0001, treatment P < 0.0001; post hoc tests wt vs. ko for the following conditions: fAβ42‐mAb11 5 μg/ml P = 0.0449, fAβ42‐mAb11 10 μg/ml P = 0.0370, fAβ42‐mAb11 20 μg/ml P = 0.0299, fAβ42‐mAb11 50 μg/ml P = 0.0120).
Quantification of relative fAβ42 uptake to lowest antibody concentration used (n = 5, ± SEM).
was normalized to wt without antibody. Bonferroni‐corrected pair‐wise
tests were used.