Abstract
Background and Aims:
To evaluate analgesic activity and safety of single oral dose (1000 mg) of Terminalia chebula using a mechanical pain model in healthy human volunteers.
Material and Methods:
Twelve healthy volunteers were randomized to receive either single oral dose of 2 capsules of T. chebula 500 mg each or identical placebo capsules in a double-blinded manner. Mechanical pain was assessed using Ugo basile analgesy meter (Randall–Selitto test) before and 3 h after administration of test drug. The parameters evaluated were pain threshold force and time; pain tolerance force and time. A washout period of 1-week was given for crossover between active drug and placebo.
Results:
Terminalia chebula significantly increased the mean percentage change for pain threshold force and time, and pain tolerance force and time compared to placebo (P < 0.001). The mean percentage change for pain threshold force and time (20.8% and 21.0%) was increased more than that of pain tolerance force and time (13.4% and 13.4%). No adverse drug reaction was reported with either of the study medications during the study period.
Conclusion:
T. chebula significantly increased pain threshold and pain tolerance compared to placebo. Both the study medications were well tolerated. Further multiple dose studies may be needed to establish the analgesic efficacy of the drug in patients suffering from osteoarthritis, rheumatoid arthritis and other painful conditions.
Key words: Pain threshold force and time, pain tolerance force and time, Terminalia chebula, Ugo basile analgesy meter (Randall–Selitto test)
Introduction
Despite the enormous advances in drug development over the past two decades, development of novel analgesics has lagged behind. Analgesics that are used widely are opioids and nonsteroidal anti-inflammatory agents (NSAIDs), all of which have potentially threatening toxicities even when used in therapeutic doses. Their adverse effects and insufficient effectiveness in many types of pain are the main driving forces in the development of new analgesics.[1] The main factors contributing to the apparent draught of novel analgesics are insufficient mechanism-based approach to clinical pain syndromes and inadequate predictive validity of animal models of pain in humans. In addition, the neurobiology of nociceptive systems differs between species, and this limits the extrapolation of findings from animal studies to man even further. Experimental pain models in healthy volunteers for evaluation of analgesic action minimize the gap between knowledge gained in animal and human clinical studies.[2]
Among several experimental approaches in humans for early screening of new analgesics, experimentally-induced mechanical pain model is associated with good precision and reproducibility. Commonly, these tests measure subjective pain after induction of pain stimuli. The method originally described by Randall and Selitto has been used by many investigators and has been proven to detect the efficacy of analgesics.[3] Ugo basile analgesy meter (Randall–Selitto test) was used to measure the analgesic property in the present study as Terminalia chebula was found to have analgesic and anti-inflammatory properties probably mediated by the peripheral and central mechanisms.[4,5] Preliminary studies by Shailasree et al.[6] have indicated anti-inflammatory activity for the aqueous extracts of fruits of T. chebula and could inhibit cyclooxygenase-1 (COX-1), COX-2, 5-lipoxygenase (5-LOX), tumor necrosis factor-alpha (TNF-α), and down-regulation of nuclear factor-kappa B.[6]
In the present study, we have evaluated the effect of 1000 mg of aqueous extract of T. chebula on pain in healthy human volunteers using a mechanical pain model.
Material and Methods
The present study was approved by the Institutional Ethics Committee and was performed as per the Good Clinical Practice and declaration of Helsinki guidelines. This was a randomized, double-blind, placebo-controlled, cross-over study. Twelve healthy male volunteers aged 18-40 years, with a body mass index in the range of 19.5-25.9 kg/m2 participated in the study. The study medication consisted of two capsules (500 mg each) of T. chebula and two placebo capsules, both given as single dose in a randomized manner. Each capsule of T. chebula contained a patented standardized aqueous extract of T. chebula with ≥ 15% of chebulinic acid, ≥ 10% of chebulagic acid and ≥ 15% of other low molecular weight hydrolysable tannins and is highly standardized by high performance liquid chromatography. Placebo capsules were identical to T. chebula capsules in size, shape, and color and were supplied by the study sponsor, Natreon Inc., New Jersey, USA.
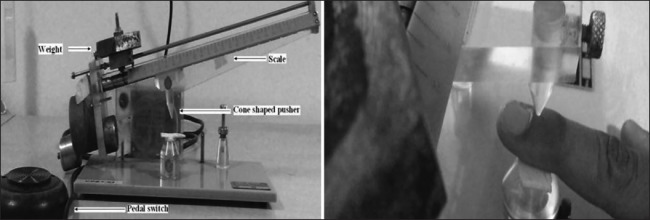
The volunteers were screened for eligibility into the study. The successfully screened healthy volunteers were trained on two separate occasions for the Randall–Selitto test procedure prior to participation into study. In the present study, we evaluated the analgesic activity of T. chebula using mechanical pain model by Randall–Selitto test using analgesy meter (Ugo-Basile, Biological Research Apparatus, Milan, Italy). Participant was asked to sit comfortably on a chair with his eyes blindfolded. Participant's nail bed of index finger of nondominant hand was placed on a small plinth of basile analgesy meter, under a cone shaped pusher with a rounded tip as shown in Figure 1. A pedal switch was pressed by the investigator to apply force to the nail bed. The instrument is geometrically designed to increase the force on the finger at a constant rate of 80 g/s. The participant was asked to lift his index finger of the other hand when he first experiences pain (noted as pain threshold) and when he was unable to tolerate the pain (noted as pain tolerance). Then, pressure on the pedal switch was removed, and the weight-bearing arm of the instrument was lifted to release the pressure on the nail bed. Readings on the scale were recorded. Three readings were taken at 5 min intervals. The average of the three readings was taken and the force (in grams) exerted on the nail bed and the threshold time and tolerance time were calculated.
Figure 1.
Components of Ugo basile analgesy meter
The volunteers were given either two capsules of 500 mg T. chebula or identical looking placebo capsules with 240 ml of water at 8:00 AM as per prior randomization schedule (run 1), in a double-blind manner. After taking the drug, the subject was asked to sit upright on the chair for 2 h. Randall–Selitto test was repeated 3 h after the drug administration and the readings were noted. After 2 weeks of washout period, the second drug (run 2) was administered according to the randomization sequence and the same procedure was repeated. All safety lab parameters were repeated after administration of both drugs.
Paired and unpaired Student's t-test were used to assess the analgesic efficacy of T. chebula with P < 0.05 considered to be statistically significant. All statistical analyses were performed using the GraphPad PRISM software 4 (GraphPad Software Inc., USA).
Results
14 subjects were screened for participation with two excluded due to hypertension. The mean age of the volunteers was 32.3 ± 3.1 years, and average body mass index was 22.4 ± 1.0 kg/m2.
A statistically significant increase in mean pain threshold force, mean pain threshold time, mean pain tolerance force and mean pain tolerance time was observed with T. chebula with a P <0.001. The increase in mean pain threshold force, mean pain threshold time, mean pain tolerance force and mean pain tolerance time observed with placebo was statistically nonsignificant [Table 1].
Table 1.
Pain threshold force and time; pain tolerance force and time with T. chebula and placebo at baseline and postdrug
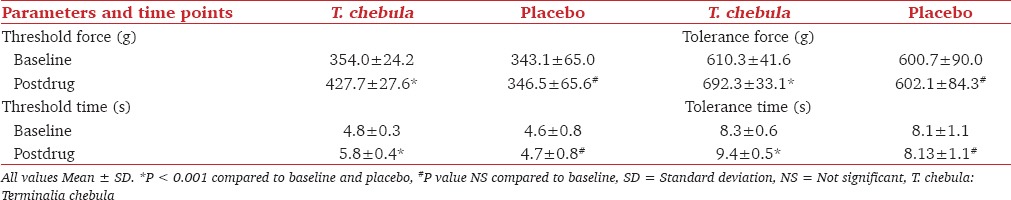
When compared to baseline, there was 20.8% increase in pain threshold force postdrug with T. chebula, and 1.0% with placebo. Whereas 20.1% increase in pain threshold time was observed with T. chebula, and 0.86% with placebo. Pain tolerance force postdrug increased by 13.4% with T. chebula, and 0.2% with placebo. Whereas 13.4% increase in pain tolerance time was observed with T. chebula, and 0.2% with placebo.
Compliance was assured as the drugs were administered under supervision. Both the drugs were well tolerated, and none of the subjects reported any adverse events. All safety lab investigations were done before and after administration of each drug and were found to be normal.
Discussion
In the present study, aqueous extract of T. chebula has demonstrated analgesic activity in healthy human volunteers using experimentally-induced mechanical pain by showing a significant increase in the pain threshold force and time; pain tolerance force and time compared to placebo. T. chebula is widely used in Ayurvedic medicine and found to have anti-inflammatory and analgesic effect by inhibition of COX-1, COX-2, 5-LOX, TNF-α and down-regulation of NF-κB.[7]
Terminalia chebula showed analgesic, anti-pyretic and anti-inflammatory properties without producing gastric ulceration in rats.[8] Kaur and Jaggi[9] evaluated the extracts of T. chebula fruit for their analgesic activity using the tail immersion model in mice exhibiting analgesic response at 200, 400 and 800 mg/kg body weight in acute pain and in chronic pain with maximum analgesic response on 14th day. The results suggested that T. chebula could be a potential candidate for bioactivity-guided isolation of natural analgesic agents in the management of chronic pain.[9]
In another study by Nair et al.,[7] hydroalcoholic extract of T. chebula produced a significant inhibition of joint swelling compared to control in both formaldehyde-induced and complete Freund's adjuvant-induced arthritis in mice. T. chebula treatment also reduced serum TNF-α level and synovial expression of TNF-R1, interleukin-6 (IL-6) and IL-1β thereby exhibiting its therapeutic potential as a disease-modifying agent in the treatment of rheumatoid arthritis. In a study by Ramani and Pradhan[10] acetone extract of fruits of T. chebula in doses of 160 and 320 mg/kg showed anti-inflammatory and antiarthritic effects as it decreased the paw edema significantly (P < 0.05) which was comparable to dexamethasone treated group. Also, in contrast to the traditional NSAIDs, which are reported to produce gastric ulceration and gastrointestinal bleeding, T. chebula extract is reported to have gastro protective effect.[10]
To the best of our knowledge, there is limited published data on evaluation of the analgesic activity of T. chebula alone in human subjects. In the present study, we used Ugo basile analgesy meter for conducting Randal–Selitto test as it is associated with good precision and smaller inter-individual and intra-individual variation of baseline effect. In the present study, aqueous extract of T. chebula 1000 mg given as single dose was well tolerated without producing any adverse effects.
Conclusion
T. chebula significantly increased pain threshold and pain tolerance force and time compared to placebo in healthy volunteers. Both the study medications were well tolerated, and no adverse events were observed during the study. Further multiple dose studies may be needed to establish the analgesic efficacy of the drug in patients suffering from osteoarthritis, rheumatoid arthritis and other painful conditions which require analgesics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank ICMR Advanced centre for Clinical Pharmacodynamics, NIMS, Hyderabad for developing pain models, Natreon Inc, NJ, USA for supplying the study medications and Dr. I. Shravanthi, Ayurvedic physician for her expert advice regarding the study.
References
- 1.Kissin I. The development of new analgesics over the past 50 years: A lack of real breakthrough drugs. Anesth Analg. 2010;110:780–9. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- 2.Olesen AE, Andresen T, Christrup LL, Upton RN. Translational pain research: Evaluating analgesic effect in experimental visceral pain models. World J Gastroenterol. 2009;15:177–81. doi: 10.3748/wjg.15.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhavathi K, Chandra US, Soanker R, Rani PU. A randomized, double blind, placebo controlled, cross over study to evaluate the analgesic activity of Boswellia serrata in healthy volunteers using mechanical pain model. Indian J Pharmacol. 2014;46:475–9. doi: 10.4103/0253-7613.140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samad MB, D’Costa NM, Kabir AU, Hannan JM. Investigation on central and peripheral analgesic and anti-inflammatory activity of Punarnavasava, an Ayurvedic preparation. Br J Pharm Res. 2013;3:146–62. doi: 10.4103/0976-0105.105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashwini R, Gajalakshmi S, Mythili S, Sathiavelu A. Terminalia chebula — A pharmacological review. J Pharm Res. 2011;4:2884–7. [Google Scholar]
- 6.Shailasree S, Ruma K, Kini KR, Niranjana SR, Prakash HS. Potential anti-inflammatory bioactives from medicinal plants of Western Ghats, India. Pharmacogn Commun. 2012;2:2–12. [Google Scholar]
- 7.Nair V, Singh S, Gupta YK. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J Pharm Pharmacol. 2010;62:1801–6. doi: 10.1111/j.2042-7158.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Prakash T, Kotresha D, Ansari MA, Sahrm UR, Kumar B, et al. Antiulcerogenic activity of Terminalia chebula fruit in experimentally induced ulcer in rats. Pharm Biol. 2011;49:262–8. doi: 10.3109/13880209.2010.503709. [DOI] [PubMed] [Google Scholar]
- 9.Kaur S, Jaggi RK. Antinociceptive activity of chronic administration of different extracts of Terminalia bellerica Roxb. and Terminalia chebula Retz. fruits. Indian J Exp Biol. 2010;48:925–30. [PubMed] [Google Scholar]
- 10.Ramani YR, Pradhan S. Antiarthritic activity of acetone extract of Terminalia chebula. Webmedcentral Pharmacol. 2012;3:WMC003057. [Google Scholar]