Abstract
Background and Aims:
Laparoscopic pediatric surgery allows a rapid postoperative rehabilitation and hospital discharge. However, the optimal postoperative pain management preserving advantages of this surgical technique remains to be determined. This study aimed to identify factors affecting the postoperative recovery of bowel function after laparoscopic surgery in children.
Material and Methods:
A retrospective analysis of factors affecting recovery of bowel function in children and infants undergoing laparoscopic surgery between January 1, 2009 and September 30, 2009, was performed. Factors included were: Age, weight, extent of surgery (extensive, regional or local), chronic pain (sickle cell disease or chronic intestinal inflammatory disease), American Society of Anaesthesiologists status, postoperative analgesia (ketamine, morphine, nalbuphine, paracetamol, nonsteroidal anti-inflammatory drugs [NSAIDs], nefopam, regional analgesia) both in the Postanesthesia Care Unit and in the surgical ward; and surgical complications. Data analysis used classification and regression tree analysis (CART) with a 10-fold cross validation.
Results:
One hundred and sixty six patients were included in the analysis. Recovery of bowel function depended upon: The extent of surgery, the occurrence of postoperative surgical complications, the administration of postoperative morphine in the surgical ward, the coadministration of paracetamol and NSAIDs and/or nefopam in the surgical ward and the emergency character of the surgery. The CART method generated a decision tree with eight terminal nodes. The percentage of explained variability of the model and the cross validation were 58% and 49%, respectively.
Conclusion:
Multimodal analgesia using nonopioid analgesia that allows decreasing postoperative morphine consumption should be considered for the speed of bowel function recovery after laparoscopic pediatric surgery.
Key words: Bowel function, laparoscopy, pediatrics, postoperative morphine
Introduction
Laparoscopic surgery is associated with a decrease of postoperative pain and surgical complications and a faster postoperative rehabilitation.[1] This technique has also become the standard surgical technique for many pediatric interventions, previously realized by open laparotomy.[2] The miniaturization of optical and dissection devices used during this surgery and the increase in skills of many surgical teams have greatly contributed to the development of this technique in pediatric abdominal and urological surgeries.[3] Pediatric laparoscopic surgery also involves a faster postoperative rehabilitation.[4,5] This includes a rapid recovery of bowel function (that allows oral treatment administration), a rapid removal of catheters (intravenous, bladder etc.,) active pain treatment and rapid mobilization. However, appropriate postoperative analgesia management during this kind of surgery and its influence on postoperative rehabilitation, especially the recovery of bowel function, has not yet been investigated.
The goal of the present study was to assess the influence of postoperative pain management, patient's characteristics (demographic and health status) and surgery performed on bowel function recovery after pediatric laparoscopic surgery. Bowel function recovery is meaningful in this context as one of the main criteria for postoperative rehabilitation.
Material and Methods
The study consists in a retrospective analysis of data from children operated in our institution between January 1, 2009 and September 30, 2009. Patients were selected from our electronic hospitalization billing database and data were extracted by three anesthesiologists from paper records. This study was approved by our local ethical committee (IRB of Robert Debré Hospital: #2012/28). The requirement for written informed consent was waived by the institutional review board.
All patients operated using laparoscopic techniques without laparotomy conversion were selected for this study. Anesthesia was provided according to our local protocols. All patients underwent a 3 min preoxygenation. In the case of emergency surgery or intestinal occlusion a rapid sequence induction using propofol (2-3 mg/kg) or thiopental (5-7 mg/kg), and succinylcholine (2 mg/kg) was performed. Otherwise induction was performed using sevoflurane (6% in a mixture of O2/N2O 50%/50%) or intravenous propofol (5-7 mg/kg). All patients were mechanically ventilated after the trachea was intubated and a nondepolarizing muscle relaxant was systemically given in order to facilitate both intubation and surgical procedure (atracurium 0.5 mg/kg at induction). Muscle relaxation was systemically antagonized at the end of surgery. All patients were given intraoperative opioids (sufentanil: 0.2 µg/Kg at induction and 0.1 µg/kg when intraoperative heart rate or mean arterial pressure increased above 20% of the preoperative value). Prevention of postoperative nausea and vomiting began after induction using both dexamethasone (0.15 mg/kg) and ondansetron (0.1 mg/kg). Postoperative analgesics were selected by individual anesthesiologists (intraoperative period and Postanesthesia Care Unit [PACU]) and surgeons (surgical ward) among opioid (morphine and nalbuphine) and nonopioid analgesics (paracetamol, nonsteroidal anti-inflammatory drugs [NSAIDs] and nefopam). Morphine was administered by intravenous titration in the PACU and by Patient or Nurse Controlled Analgesia in the PACU and the surgical ward. Otherwise, patients were given nalbuphine after morphine titration. Nalbuphine was administered as a bolus of 0.2 mg/kg followed by continuous administration (1 mg/kg/day). During hospital stay, morphine was either administered as a first line pain treatment or as a rescue therapy if nalbuphine failed to relieve pain.
Morphine administration was evaluated on the second postoperative day and replaced by a continuous intravenous administration of nalbuphine (1 mg/kg/day) if no bolus was administered. Consistent with our protocols, intravenous opioids were prescribed during the first two postoperative days and replaced by oral codeine when nonopioid analgesics were insufficient to relieve pain.[6] Administration of nonopioid analgesics (intraoperativelly, during PACU stay or in the surgical ward) or regional analgesia, were determined by anesthesiologists at their discretion. Nonopioid analgesics were administered according to our local and national guidelines (www.pediadol.org). They included intravenous paracetamol (15 mg/kg/6 h or 7.5 mg/kg/6 h when weight was less than 10 kg), intravenous NSAIDs (Ketorolac, 1 mg/kg/8 h) and intravenous nefopam (0.25 mg/kg/6 h, for patients older than 8 years). When the patients started accepting oral feeds, they received oral paracetamol (15 mg/kg 4 times a day) and oral ibuprofen (10 mg/Kg 4 times a day). Caudal analgesia was decided and performed by the anesthesiologist caring for the patient at the end of surgery during urological procedures when appropriate. It consisted of a single bolus of levobupivacaine (2.5 mg/ml) at 1 ml/kg (maximum 20 ml). Parents were not allowed to stay with their child or infant during induction or PACU stay. In the PACU and in the surgical ward, analgesia was managed according to pain assessment. The assessment tools used were the Objective Pain Scale (OPS) for preschool children and the visual analog scale (VAS) for the older children. The goal of the analgesic management was to keep OPS or VAS under four.
Management of postoperative rehabilitation consisted of:
Rapid removal of the feeding tube (either at the end of surgery or during the first 12 postoperative h),
Oral feeding when bowel functions recovered and
Rapid discharge from hospital. Recovery of bowel function was defined by the occurrence of bowel movement or flatus. Postoperative nausea and vomiting was managed using ondansetron (0.1 mg/kg 3 times a day).
Data collected
Data collected included: Age, weight, surgery performed, chronic pain (sickle cell disease or chronic intestinal inflammatory disease), American Society of Anaesthesiologists status, postoperative analgesia in the PACU and in the surgical ward: Ketamine, morphine, nalbuphine (given in the first place or replacing morphine), NSAIDs (both intravenous or oral), nefopam, regional analgesia (either caudal analgesia, epidural analgesia or both), surgical complications and their management, time to recovery of bowel function and length of hospital stay. Recovery of bowel function was defined as the time of first feeding after flatus passage or bowel movement. Percentages of missing values were reported for each considered factor.
For the purpose of analysis the surgery was classified as local, regional or extensive according to its extent and invasiveness defined by surgeons, and the predicted associated postoperative pain as defined by the French Society of Anesthesiology and Intensive Care (http://www.sfar.org/article/21/prise-en-charge-de-la-douleur-post-operatoire-chez-l-adulte-et-l-enfant-cc-1997). Local surgery included: Simple appendectomy, testicular ectopy, herniorraphy and exploratory laparoscopy; regional surgery included: Nissen surgery, cholocystectomy, localized intraabdominal abscess (without peritonitis), nephrectomy, Duhamel operation, pyeloplasty, ovariectomy and splenectomy; extensive surgery included: Peritonitis, digestive duplication, intestinal occlusion and any combination of two or more local and/or regional surgeries.
Statistical analysis
In order to study the impact of the factors cited above on the recovery of bowel function and to classify this outcome according to those factors, we performed a classification tree analysis.[7,8,9] This statistical method seems adapted to our heterogeneous population and allows classifying the sample according to predictors and their effects on one another. In addition, classification and regression tree (CART) analysis needs neither normal distribution nor independence of variables. This allows entering in the model factors that might be statistically dependent such as importance and surgery and morphine administration, with more frequent use of morphine during extensive surgeries.
This analysis generates classification trees defined by nodes which optimally classify the population in homogeneous groups according to the influence of the considered factors on the studied outcome. The methodology relies on the dichotomization (or division) of the population according to the predictors entered in the model. Each dichotomization (node) defines two child groups where intra-group variability is minimized. The predictor associated with each node is the one which maximizes the reduction of variability (measured by the variance) in that subpopulation. Each child group undergoes further dichotomization in a recursive manner. For interpretation purposes, one may focus on the tree's terminal nodes which constitute the “groups.” Going up in the tree allows characterizing these groups according to the presence or absence of the risk factors associated with parent node in the tree.
In order to avoid excess division, the tree function was stopped when the explained variability of the studied variable stops to increase. This was assessed using the lift curve that explores the explained variability as a function of the number of divisions of the tree.
As recommended, the validity of this model was studied using the percentage of explained variability (derived from the misclassification risk of the CART model) and by cross validation. A tenfold cross validation was used in the current study Model validity was also assessed using a Pearson linear regression between predicted (determined by the model classification) and observed values of time to bowel function recovery. Statistical analysis was performed using JMP 9.0 (JMP, Version 9. SAS Institute Inc., Cary, NC, 1989-2011) and SPSS 20.0 Software (IBM Company, Chicago, Illinois, USA).
Results of CART analysis are displayed as a tree, with each node containing factors that allow dichotomization, mean ± standard deviation of time to bowel function recovery and number of patients. The correlations between observed and predicted duration of recovery of bowel function was expressed as the Spearman coefficients of correlation.
Results
One hundred and sixty six patients with complete data were included in the analysis None of them were in an ambulatory program and none discharged on the day of surgery. There were no excluded patients because of missing data. Descriptive statistics are shown in Tables 1 and 2. According to our classification, there were 45.2% local surgeries, 36.1% regional surgeries and 18.7% extensive surgeries. Except patients with preoperative chronic pain, all others (including emergent patients) did not receive preoperative opioid therapy.
Table 1.
Demographic, surgical and postoperative pain management characteristics of the population
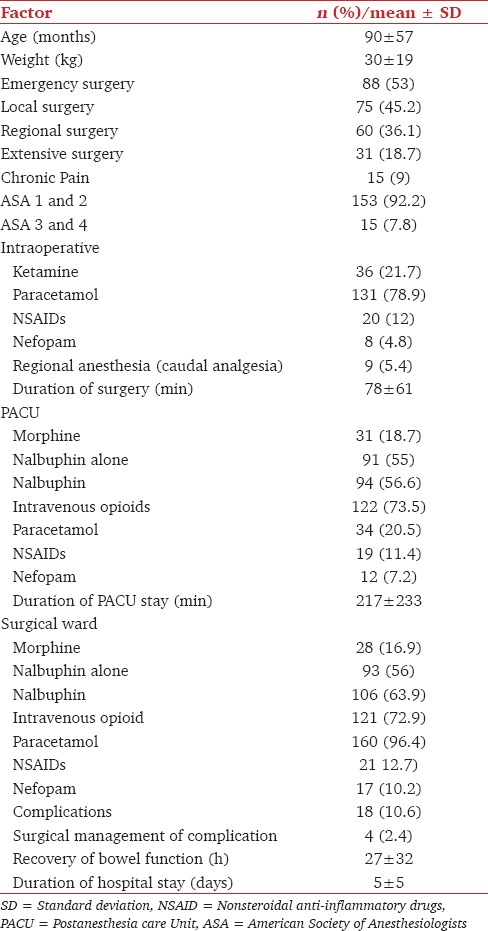
Table 2.
Demographic, surgical and postoperative pain management characteristics of the population according to the extent of surgery
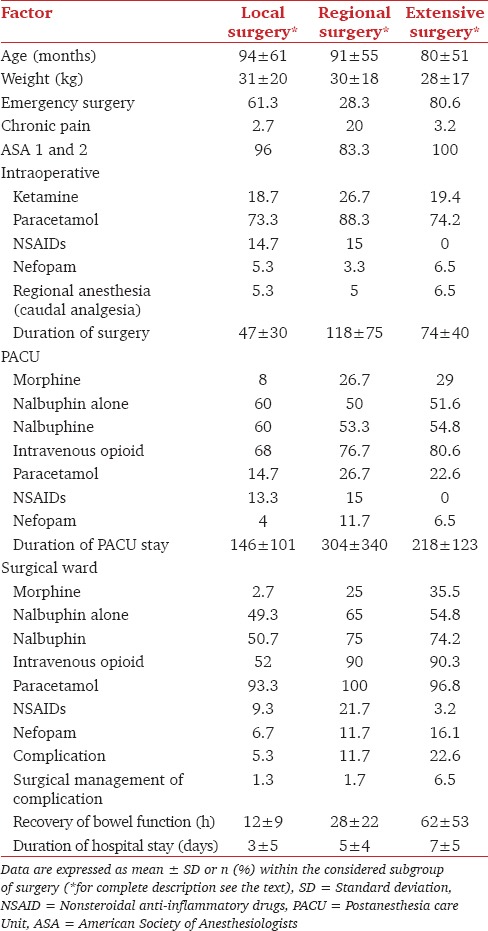
Concerning postoperative analgesia, results are displayed in Tables 1 and 2. Paracetamol was the most frequently used (20.5% and 96.4% in the PACU and the surgical ward, respectively). Consequently, this factor was not entered in the CART analysis. NSAIDs and nefopam were used less frequently but their administration increased with the invasiveness of surgery and was systematically associated with paracetamol (except for NSAIDs during extensive surgeries, Table 2). Consequently, this factor could not be analyzed independently of paracetamol administration.
Postoperative complications occurred in 18 patients. These consisted of intra-abdominal or wound abscesses (14 patients); surgical incision dehiscence (one patient), postoperative wound hyperalgesia (one patient), postoperative fever (one patient), evisceration and failure after Toupet intervention (one patient). Surgery was required in four cases: One intra-abdominal abscess, one wound abscesses, one evisceration and one intervention failure (severe dysphagia after Toupet intervention). None of those complications were related to analgesia.
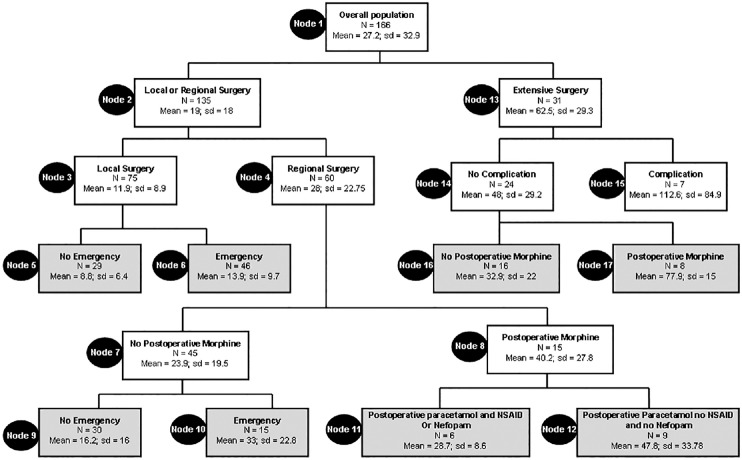
Results of CART analysis are shown in Figure 1. It generated eight terminal nodes forming eight groups of patients. According to this tree, five classifier factors were found: Extent of surgery, occurrence of postoperative surgical complication, postoperative morphine administration in the surgical ward, emergency surgery and postoperative co-administration of paracetamol-NSAIDs or paracetamol-nefopam in the surgical ward [Figure 1]. All other factors included in the analysis, including demographic data and regional analgesia, were nonsignificant and were discarded by the model. The derived tree explained 58% of the variability of the studied outcomes and the lift curve [Figure 2] indicates no excess of dichotomization as no ceiling of explained variability occurs during divisions. The tenfold validation similarly led to 49% of explained variability. Spearman correlation between observed and predicted values of time to bowel function recovery was statistically significant [Figure 3, r2= 0.75; P < 0.001].
Figure 1.
Classification and regression analysis tree. Each node contains mean and standard deviation of observed values of recovery of bowel function (expressed in hours), the number (n) of patients in the node. Bold boxes indicate terminal nodes
Figure 2.
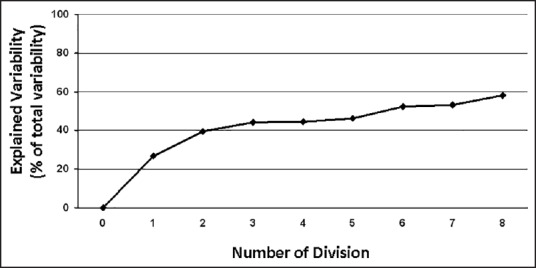
Lift curve fitting the number of division against the explained variability of the model
Figure 3.
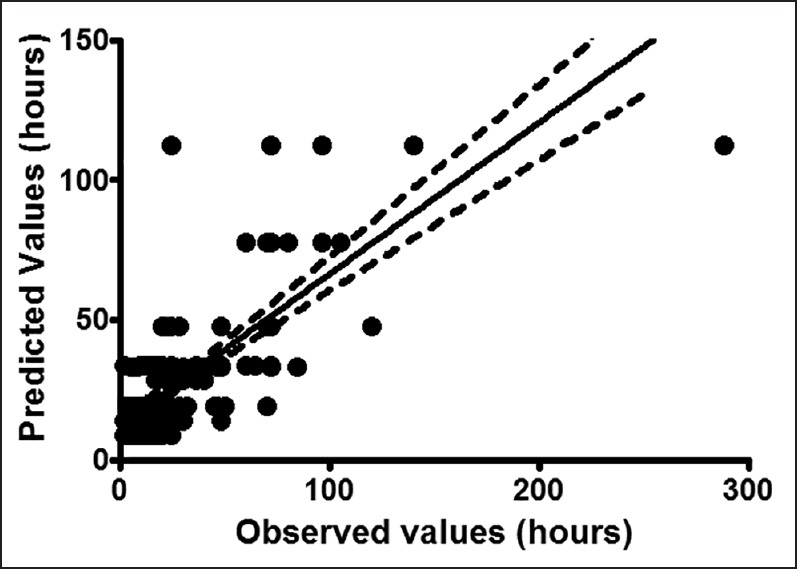
Correlation between observed and predicted individual values of recovery of bowel function (expressed in hours). Dashes lines (95% confidence interval of the regression), continuous line: Mean correlation regression
Discussion
The main findings of our study is that the time to bowel function recovery after laparoscopy in children and infants is affected by the extent of surgery, the occurrence of postoperative surgical complication, the administration of postoperative morphine, the co-administration of paracetamol and NSAIDs or nefopam and whether the surgery was performed as an emergency procedure. Our model explained 58% of the variability of the outcome with a satisfactory cross validation and an acceptable level of accuracy.
Laparoscopic surgery in children and infants has become frequently used in many centers instead of open laparotomy.[1,3,4,10] This surgical technique has been successfully used for many interventions such as appendectomy, cholecystectomy, urological surgery, colectomy or enterotomy and oncologic surgery. Many studies and meta analyses have found at least one of the following benefits: Decrease of surgical complications, rapid postoperative rehabilitation (including faster recovery of bowel function and oral intakes), a decrease of postoperative pain and faster hospital discharge.[1,3,4,10]
Postoperative pain management in children involves important factors that must be taken into account. Although morphine is used as a first line postoperative pain treatment, it is less effective in infants than in adult patients and is associated with more frequent complications such as delayed of bowel function recovery, nausea and vomiting, pruritus and urinary retention.[11] In addition, opioid-sparing compounds such as ketamine have been found ineffective in children.[12] Moreover, nonopioid analgesics, such as NSAIDs or nefopam are not fully labeled for pediatric use or only for particular ranges of age and weight, even though, they are known to produce opioid-sparing effects in adult patients.[13] Consequently, investigating factors affecting pediatric postoperative rehabilitation, especially those related with analgesia and opioid administration, is of great importance.
Among factors found to influence the recovery of bowel function after laparoscopic surgery, three were dependant on the nature of surgery (extent and invasiveness of surgery, postoperative complications, and performance as an emergency procedure) and two involved postoperative pain management (postoperative morphine consumption and nonopioid analgesics administration). Our study confirmed previous findings that postoperative morphine administration results in delayed bowel function recovery (nodes 16, 17 and 7, 8). As previously found,[14] the mixed agonist-antagonist µ opioid receptor nalbuphine was not found to be a predictor of bowel function recovery. This result must not be interpreted as an argument against the use of postoperative morphine but rather as an indication to decrease the amount of administered morphine. Decreasing postoperative morphine administration can be achieved by associating this agent with nonopioid analgesics such as paracetamol, NSAIDs and nefopam.[13] Paracetamol and NSAIDs have been previously found to induce an opioid sparing effect in children.[15,16,17] In adult patients, nefopam, an NMDA receptor antagonist, has been unambiguously shown to decrease opioid and morphine consumption during the postoperative period which might in turn decrease the time to return of bowel function.[13] Interestingly, our study is one among the first which found an advantage of this compound in children. Adding nefopam to paracetamol decreased the time to recovery of bowel function [Figure 1 Nodes 11 and 12]. Other pharmacological and nonpharmacological treatments might also be used in order to decrease morphine consumption. In adults patients intravenous lidocaine,[18] preoperative gabapentin[19,20,21] or intraperitoneal or wound local anesthetics administration[18,22,23,24] have been previously found to decrease postoperative opioid requirement and improve postoperative pain relieve. However, their use in children is limited by the lack of evidences. Our analysis did not find any benefit of regional analgesia in decreasing time to recovery of bowel function. However, the limited number of patients who underwent these techniques may explain this result.
Our study found the extent of surgery, occurrence of surgical complications and the emergency character of surgery to be predictors of delayed bowel function recovery. The impact of the extent of the surgery and reintervention is unsurprising regarding the intensity of the postoperative ileus induced by extensive surgery. The relation between emergency surgery and the recovery of bowel function is more complicated to interpret especially when considering most of emergency surgery cases were simple or locally abscessed appendicitis. One can hypothesize that perioperative psychological factors such as anxiety (from both parents and the child) might contribute to this observed delayed bowel function recovery.[25] Otherwise, preoperative opioids might have impact on the postoperative recovery of bowel function. However, this was not the case in our sample while no emergency was managed with preoperative opioids. Finally, the importance of the inflammatory response during emergency surgeries might also explain the delayed recovery of bowel function.
Our study found a satisfactory correlation between estimated and observed values of time to bowel function recovery. Moreover, the 10-fold cross validation found a similar result concerning the explained variability, in comparison to the model determination (49% vs. 58% respectively). However, the unexplained variability (42%) suggests that some factors not included in our analysis such as surgeons' skills or patient and parents-related psychological factors should be considered in future investigations.
Our study suffers some limitations. Firstly it is a retrospective study, and so the data were collected without a previous standardized prescription protocol for both opioid and nonopioid analgesics. Consequently, systemic analgesia (including opioid and nonopioid agents association) and regional analgesia was under-used. This might have decreased the quality of analgesia and the speed of postoperative rehabilitation. However, as stated earlier, results of our analyses display a model with a high degree of explained variability of the studied outcome and a good reproducibility of this model. Secondly, morphine consumption was not investigated in our study. Considering this factor would allow studying the effect of morphine consumption on postoperative bowel function recovery. However, doing so instead of studying other factors would not have added any knowledge to the previously documented dose-related effect of morphine on bowel function recovery. In addition, regarding the continuous nature of our studied outcome (time to recover the bowel function) using doses of morphine might give irrelevant result.[26] Finally, the retrospective methodology of our study might introduce report bias in morphine doses with irrelevant results concerning this factor.
Conclusion
Our study found five factors associated with time to recovery of bowel function: The extent of surgery, surgery complications, the emergency character of surgery, postoperative morphine administration and the postoperative administration of NSAIDs or nefopam (co administered with paracetamol). Multimodal analgesia using nonopioid analgesics that allows decreasing morphine consumption should be considered for postoperative rehabilitation during laparoscopic surgery in children.
Acknowledgement
Authors thank Amel Sahli, statistical PhD for her help in statistical analyses.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Li X, Zhang J, Sang L, Zhang W, Chu Z, Li X, et al. Laparoscopic versus conventional appendectomy: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2010;10:129. doi: 10.1186/1471-230X-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Hove C, Hardiman K, Diggs B, Deveney C, Sheppard B. Demographic and socioeconomic trends in the use of laparoscopic appendectomy from 1997 to 2003. Am J Surg. 2008;195:580–3. doi: 10.1016/j.amjsurg.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 3.El-Ghoneimi A, Sauty L, Maintenant J, Macher MA, Lottmann H, Aigrain Y. Laparoscopic retroperitoneal nephrectomy in high risk children. J Urol. 2000;164:1076–9. doi: 10.1097/00005392-200009020-00039. [DOI] [PubMed] [Google Scholar]
- 4.Lejus C, Delile L, Plattner V, Baron M, Guillou S, Héloury Y, et al. Randomized, single-blinded trial of laparoscopic versus open appendectomy in children: Effects on postoperative analgesia. Anesthesiology. 1996;84:801–6. doi: 10.1097/00000542-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Shaikh AR, Sangrasi AK, Shaikh GA. Clinical outcomes of laparoscopic versus open appendectomy. JSLS. 2009;13:574–80. doi: 10.4293/108680809X1258998404524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tremlett M, Anderson BJ, Wolf A. Pro-con debate: Is codeine a drug that still has a useful role in pediatric practice? Paediatr Anaesth. 2010;20:183–94. doi: 10.1111/j.1460-9592.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 7.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–81. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 8.Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8:iii. doi: 10.3310/hta8360. [DOI] [PubMed] [Google Scholar]
- 9.Marshall RJ. The use of classification and regression trees in clinical epidemiology. J Clin Epidemiol. 2001;54:603–9. doi: 10.1016/s0895-4356(00)00344-9. [DOI] [PubMed] [Google Scholar]
- 10.Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2010;10:CD001546. doi: 10.1002/14651858.CD001546.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Duedahl TH, Hansen EH. A qualitative systematic review of morphine treatment in children with postoperative pain. Paediatr Anaesth. 2007;17:756–74. doi: 10.1111/j.1460-9592.2007.02213.x. [DOI] [PubMed] [Google Scholar]
- 12.Dahmani S, Michelet D, Abback PS, Wood C, Brasher C, Nivoche Y, et al. Ketamine for perioperative pain management in children: A meta-analysis of published studies. Paediatr Anaesth. 2011;21:636–52. doi: 10.1111/j.1460-9592.2011.03566.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: Quantitative systematic review. Br J Anaesth. 2008;101:610–7. doi: 10.1093/bja/aen267. [DOI] [PubMed] [Google Scholar]
- 14.Clemens KE, Quednau I, Klaschik E. Bowel function during pain therapy with oxycodone/naloxone prolonged-release tablets in patients with advanced cancer. Int J Clin Pract. 2011;65:472–8. doi: 10.1111/j.1742-1241.2011.02634.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiller A, Meretoja OA, Korpela R, Piiparinen S, Taivainen T. The analgesic efficacy of acetaminophen, ketoprofen, or their combination for pediatric surgical patients having soft tissue or orthopedic procedures. Anesth Analg. 2006;102:1365–71. doi: 10.1213/01.ane.0000204278.71548.bf. [DOI] [PubMed] [Google Scholar]
- 16.Korpela R, Korvenoja P, Meretoja OA. Morphine-sparing effect of acetaminophen in pediatric day-case surgery. Anesthesiology. 1999;91:442–7. doi: 10.1097/00000542-199908000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Messeri A, Busoni P, Noccioli B, Murolo S, Ivani G, Grossetti R, et al. Analgesic efficacy and tolerability of ketoprofen lysine salt vs paracetamol in common paediatric surgery. A randomized, single-blind, parallel, multicentre trial. Paediatr Anaesth. 2003;13:574–8. doi: 10.1046/j.1460-9592.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim TH, Kang H, Hong JH, Park JS, Baek CW, Kim JY, et al. Intraperitoneal and intravenous lidocaine for effective pain relief after laparoscopic appendectomy: A prospective, randomized, double-blind, placebo-controlled study. Surg Endosc. 2011;25:3183–90. doi: 10.1007/s00464-011-1684-3. [DOI] [PubMed] [Google Scholar]
- 19.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: A combined systematic review and meta-analysis. Anesth Analg. 2012;115:428–42. doi: 10.1213/ANE.0b013e318249d36e. [DOI] [PubMed] [Google Scholar]
- 20.Ghai A, Gupta M, Hooda S, Singla D, Wadhera R. A randomized controlled trial to compare pregabalin with gabapentin for postoperative pain in abdominal hysterectomy. Saudi J Anaesth. 2011;5:252–7. doi: 10.4103/1658-354X.84097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panah Khahi M, Yaghooti AA, Marashi SH, Nadjafi A. Effect of pre-emptive gabapentin on postoperative pain following lower extremity orthopaedic surgery under spinal anaesthesia. Singapore Med J. 2011;52:879–82. [PubMed] [Google Scholar]
- 22.Boddy AP, Mehta S, Rhodes M. The effect of intraperitoneal local anesthesia in laparoscopic cholecystectomy: A systematic review and meta-analysis. Anesth Analg. 2006;103:682–8. doi: 10.1213/01.ane.0000226268.06279.5a. [DOI] [PubMed] [Google Scholar]
- 23.Park YH, Kang H, Woo YC, Park SG, Baek CW, Jung YH, et al. The effect of intraperitoneal ropivacaine on pain after laparoscopic colectomy: A prospective randomized controlled trial. J Surg Res. 2011;171:94–100. doi: 10.1016/j.jss.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Stuhldreher JM, Adamina M, Konopacka A, Brady K, Delaney CP. Effect of local anesthetics on postoperative pain and opioid consumption in laparoscopic colorectal surgery. Surg Endosc. 2012;26:1617–23. doi: 10.1007/s00464-011-2079-1. [DOI] [PubMed] [Google Scholar]
- 25.Kain ZN, Caldwell-Andrews AA, Mayes LC, Weinberg ME, Wang SM, MacLaren JE, et al. Family-centered preparation for surgery improves perioperative outcomes in children: Arandomized controlled trial. Anesthesiology. 2007;106:65–74. doi: 10.1097/00000542-200701000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 1 — Pharmacokinetics. Paediatr Anaesth. 1997;7:5–11. doi: 10.1046/j.1460-9592.1997.d01-30.x. [DOI] [PubMed] [Google Scholar]