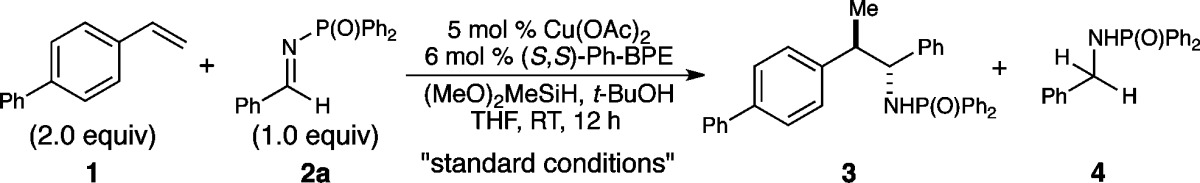
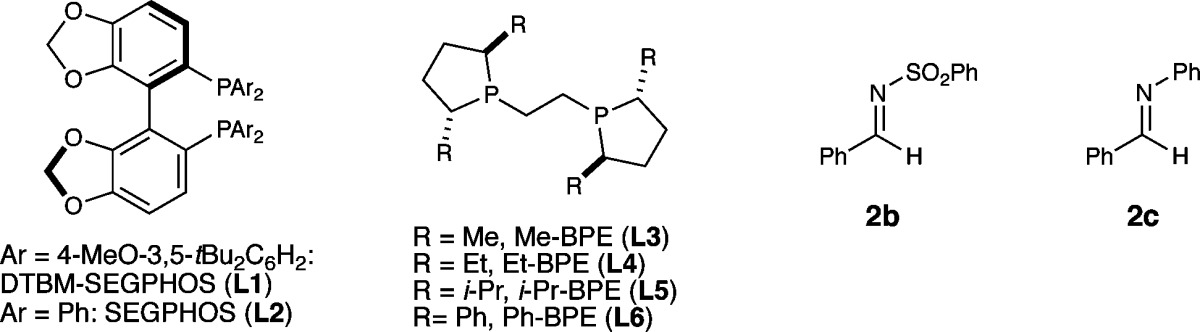
Table 1. Copper-Catalyzed Asymmetric Addition of Styrene-Derived Nucleophiles to Imines: Effect of Reaction Parametersa.
| entry | variation from the “standard conditions” | yield of 3 (dr) | ee of 3 | yield of 4 |
|---|---|---|---|---|
| 1 | none | 95% (3:1) | 99% ee (99% ee)b | 1.5% |
| 2 | L1 instead of L6 | 9% (1:1) | 95% ee (99% ee) | 85% |
| 3 | L2 instead of L6 | <5% | n.d. | 65% |
| 4 | L3 instead of L6 | <5%c | n.d. | 20% |
| 5 | L4 instead of L6 | 56% (1:1) | 86% ee (83% ee) | 35% |
| 6 | L5 instead of L6 | 46% (1:1) | 78% ee (70% ee) | 54% |
| 7 | no t-BuOH | 41% (3:1) | 99% ee (99% ee) | 31% |
| 8 | 2b instead of 2a | 5%d | n.d. | 36% |
| 9 | 2c instead of 2a | 6%e | n.d. | <10% |
Reaction conditions: 1 (0.2 mmol), 2 (0.1 mmol), Cu(OAc)2 (5 mol %), L (6 mol %), t-BuOH (2 equiv), (MeO)2MeSiH (5 equiv), THF (0.5 M), rt, 12 h. Yields were determined by 1H NMR spectroscopic analysis using 1,3,5-trimethoxybenzene as the internal standard. Enantiomeric excess values were determined by chiral HPLC analysis.
The ee of the minor diastereomer is shown in parentheses.
30% conv. of 2a.
41% conv. of 2b.