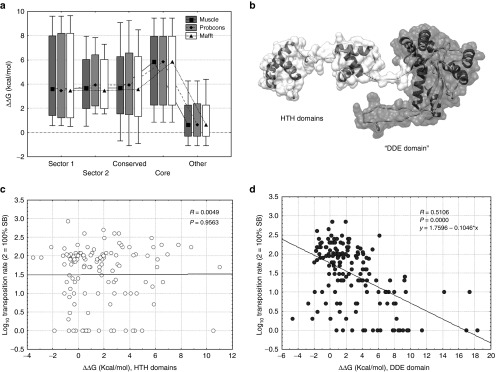
Figure 4.
The effect of mutations on the change of the free energy of unfolding (median, box: 25–75%, whiskers: 10–90%). (a) Mutations in sectors and the core are significantly more destabilizing (ΔΔG > 0) than mutants of other residues (P < 0.05 for all comparisons, t-tests). (b) The monomer of SB. The flexible N-terminal arm of the protein containing the HTH domains (residues 1–120) is indicated with white, the globular part (residues 121–340), which contains the DDE domain, with gray. (c) In the flexible arm the effect of mutations on ΔΔG is not correlated with transposition rate (P = 0.95). (d) In the globular region we find a significant negative correlation (P << 0.001, R = −0.51) between ΔΔG and transposition rate.