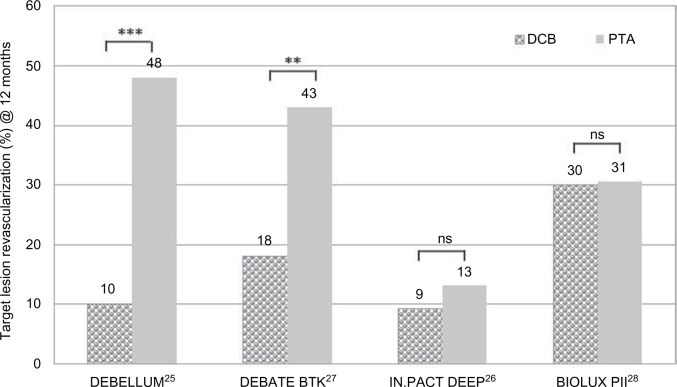
Figure 4.
Comparison of TLR at 12 months in clinical trials of DCB vs PTA in BTK lesions.
Notes: **P≤0.01; ***P≤0.001. DEBELLUM, Lower Limb Multilevel Treatment with Drug-Eluting Balloon Trial; DEBELLUM*1, 75% ATK and 25% BTK; DEBATE-BTK, Drug-Eluting Balloon in Peripheral Intervention for Below the Knee Angioplasty Evaluation Trial; IN.PACT DEEP, Study of IN.PACT Amphirion™ Drug Eluting Balloon vs Standard PTA for the Treatment of Below the Knee Critical Limb Ischemia; BIOLUX-PII, BIOTRONIK’s First-in-Men Study of the Passeo-18 LUX Drug Releasing PTA Balloon Catheter vs the Uncoated Passeo 18 Balloon Catheter in Subjects Requiring Revascularization of Infrapopliteal Arteries.
Abbreviations: BTK, below the knee; DCB, drug-coated balloon; ns, not significant; PTA, percutaneous transluminal angioplasty; TLR, target lesion revascularization.