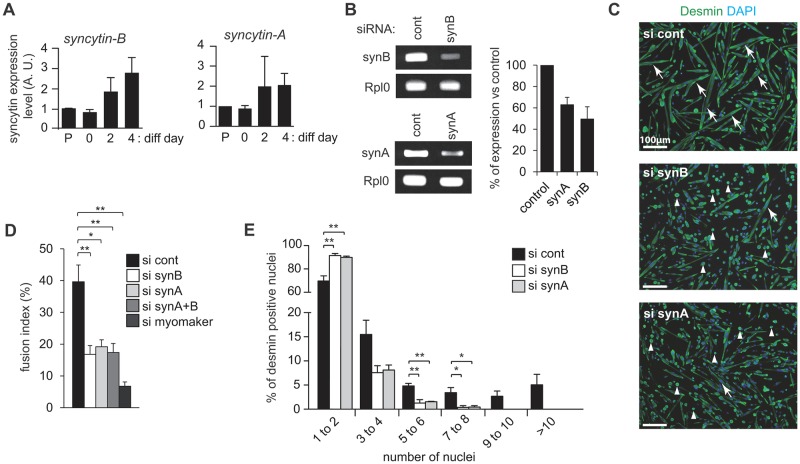
Fig 5. Murine syncytin expression and impact of their knockdown on myoblast cell-cell fusion ex vivo.
Primary myoblast cells were transfected with control siRNA (si cont) or siRNAs specifically designed against syncytin-B (si synB),–A (si synA) or myomaker (si myomaker), and differentiation was induced 2 days later. Following two days of differentiation, cells were fixed in 4% PFA, stained with an anti-desmin antibody (muscle cell marker), and nuclei were counterstained with DAPI. (A) Level of expression of syncytin-B and -A (arbitrary units, A. U.) during myoblast fusion in murine primary myoblasts, either actively proliferating (P: proliferating non confluent cells, D0: proliferative confluent cells), or in differentiation and fusion (D2 to D4), as analyzed by quantitative RT-PCR. All quantifications were normalized by RPL0, and the fold-change in gene expression expressed relative to the values of proliferating myoblasts, arbitrarily settled to one. (B) Efficiency of siRNA knockdown measured by quantitative RT-PCR. (C) Representative images of desmin- and DAPI-labelled myoblast cells transfected with control, syncytin-B or syncytin-A siRNAs. Representative myotubes are indicated by white arrows whereas white triangles correspond to mononucleated myoblast cells. (D) Fusion index (number of nuclei in cells with ≥2 nuclei per total number of nuclei). (E) Nuclei distribution per myotube. Data are the mean ± SEM (four independent experiments; * p<0.05, ** p<0.01, Mann and Whitney test).