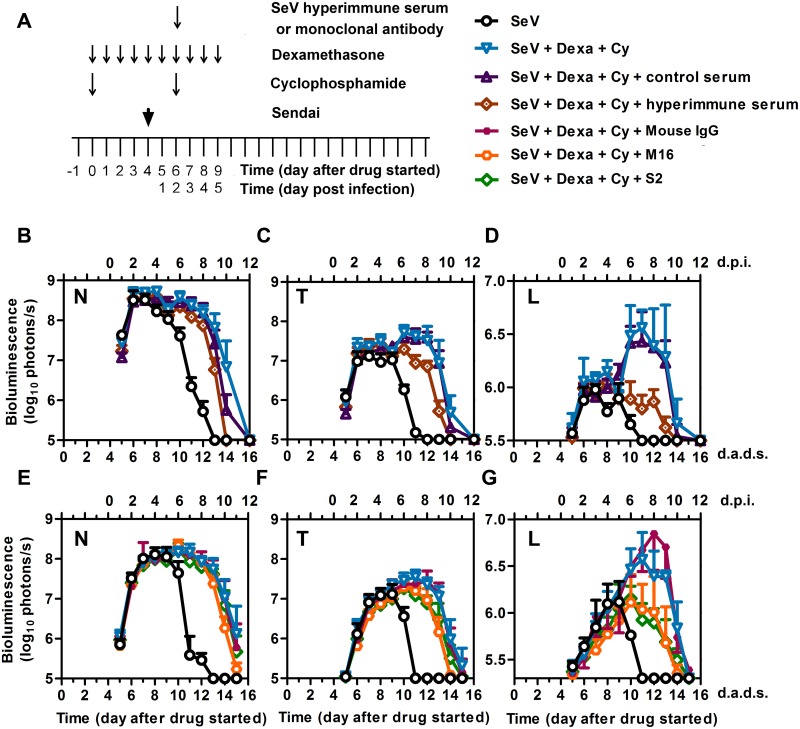
Fig 10. Effects of SeV-specific hyperimmune pooled serum and monoclonal antibodies on controlling SeV infection in immunosuppressed mice.
(A) Drug treatment regimen with SeV-specific hyperimmune serum or mAbs. Arrows denote days on which serum or monoclonal antibodies were i.p. injected, drug injections were performed, and 7,000 PFU Sendai virus (SeV) was intranasally inoculated in 5 μL PBS. (B–D) Bioluminescence in the nasopharynx (B), trachea (C), and lungs (D) after infection with SeV and the following conditions: no immunosuppression and no hyperimmune serum (black circles), Dexa + Cy without hyperimmune serum (blue triangles), Dexa + Cy with hyperimmune serum (brown diamonds), and Dexa + Cy with nonspecific control serum (dark purple triangles). (E-G) Bioluminescence in the nasopharynx (E), trachea (F), and lungs (G) after infection with SeV and the following conditions: no immunosuppression and no monoclonal antibodies (black circles), Dexa + Cy without monoclonal antibodies (blue triangles), Dexa + Cy + M16 [anti-F protein monoclonal antibody] (orange circles), Dexa + Cy + S2 [anti-HN protein monoclonal antibody] (green diamonds), and Dexa + Cy + isotype control mouse IgG (small purple squares). The data shown are averages of 5 mice per group. In all graphs, error bars represent the standard deviation. d.p.i., days postinfection; d.a.d.s., days after drug started.