Abstract
Widely distributed osteosclerosis is an unusual radiographic finding with multiple causes. A 42-year-old pre-menopausal Spanish woman gradually acquired dense bone diffusely affecting her axial skeleton and focally affecting her proximal long bones. Systemic lupus erythematosus diagnosed in adolescence had been well controlled. She had not fractured or received antiresorptive therapy, and was hepatitis C virus antibody negative. Family members had low bone mass. Lumbar spine BMD measured by dual-photon absorptiometry at age 17 years, while receiving glucocorticoids, was 79% the average value of age-matched controls. From ages 30 to 37 years, DXA BMD z-scores steadily increased in her lumbar spine from +3.8 to +7.9, and femoral neck from −1.4 to −0.7. Serum calcium and phosphorus levels were consistently normal, 25OHD <20 ng/mL, and PTH sometimes slightly increased. Her reduced eGFR was 38–55 mls/min. Hypocalciuria likely reflected positive mineral balance. During increasing BMD, turnover markers (serum bone-ALP, PINP, osteocalcin, and CTX, and urinary NTX) were 1.6- to 2.8-fold above the reference limits. Those of bone formation seemed increased more than those of resorption. FGF-23 was slightly elevated, perhaps from kidney disease. Serum OPG and TGFβ1 levels were normal, but sclerostin (SOST) and RANKL were elevated. Serum multiplex biomarker profiling confirmed a high level of SOST and RANKL, whereas DKK-1 seemed low. Matrix metalloproteinases-3 and −7 were elevated. Iliac crest biopsy revealed tetracycline labels, no distinction between thick trabeculae and cortical bone, absence of peritrabecular fibrosis, few osteoclasts, and no mastocytosis. Then, for the past three years, BMD z-scores steadily decreased. Skeletal fluorosis, mastocytosis, myelofibrosis, hepatitis C-associated osteosclerosis, multiple myeloma, and aberrant phosphate homeostasis did not explain her osteosclerosis. Mutation analysis of the LRP5, LRP4, SOST, and osteopetrosis genes was negative. Microarray showed no notable copy number variation. Perhaps her osteosclerosis reflected an interval of autoimmune-mediated resistance to SOST and/or RANKL.
Keywords: autoimmunity, bone turnover markers, lupus, matrix metalloproteinases, RANKL, sclerosing bone, sclerostin
II) Introduction
Widely distributed osteosclerosis is an unusual radiographic finding with multiple causes (Supplementary Appendix, Table 1).(1-3) In adults, the most likely explanation is metastatic disease within the skeleton, especially prostate cancer in men and breast cancer in women.(2-6) Other relatively common etiologies include marrow-centric disorders like myelofibrosis or mastocytosis.(1,3,5) Hematologic, metabolic, and infectious disorders are further possibilities.(1,3,7)
Table 1.
Sequential DXA Spine and Hip Findings
| DXA Site & Parameter | Year (patient age, yrs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year Age (Yrs) | Normal BMD | Increased BMD Peak | ||||||||
| 1990* (17) | 1991* (18) | 2003 (30) | 2005 (32) | 2007 (34) | 2009 (36) | 2010 (37) | 2012 (39) | 2014 (41) | 2015 (42) | |
| L1 – L4 Spine | ||||||||||
| g/cm2 | 0.957 | 0.939 | 1.577 | 1.693 | 1.895 | 2.005 | 2.070 | 2.001 | 1.878 | 1.776 |
| BMC (g) | - | 31.9 | 69 | 74.9 | 85.3 | 91 | 94.1 | 90.9 | 84.9 | 80.4 |
| Area (cm2) | - | 33.97 | 43.8 | 44.2 | 45 | 45.4 | 45.5 | 45.4 | 45.2 | 45.3 |
| Z-score | - | - | +3.8 | +4.8 | +6.4 | +7.3 | +7.9 | +7.3 | +6.4 | +5.6 |
| Femoral Neck | ||||||||||
| g/cm2 | - | - | 0.762 | 0.732 | 0.798 | 0.815 | 0.844 | 0.820 | 0.864 | 0.813 |
| BMC (g) | - | - | 3.5 | 3.6 | 3.7 | 3.8 | 3.8 | 3.7 | 3.9 | 3.7 |
| Area (cm2) | - | - | 4.6 | 4.9 | 4.7 | 4.6 | 4.6 | 4.5 | 4.5 | 4.6 |
| Z-score | - | - | −1.4 | −1.7 | −1.1 | −0.9 | −0.7 | −0.8 | −0.4 | −0.8 |
| Total Hip | ||||||||||
| g/cm2 | - | - | 0.828 | 0.834 | 0.939 | 0.965 | 1.028 | 1.004 | 1.000 | 0.985 |
| BMC (g) | - | - | 22.6 | 21.8 | 25.6 | 26.8 | 28.2 | 27.8 | 27.6 | 27.4 |
| Area (cm2) | - | - | 27.3 | 26.1 | 27.3 | 27.7 | 27.4 | 27.7 | 27.6 | 27.8 |
| Z-score | - | - | −1.1 | −1.0 | −0.1 | +0.1 | +0.6 | +0.5 | +0.4 | +0.3 |
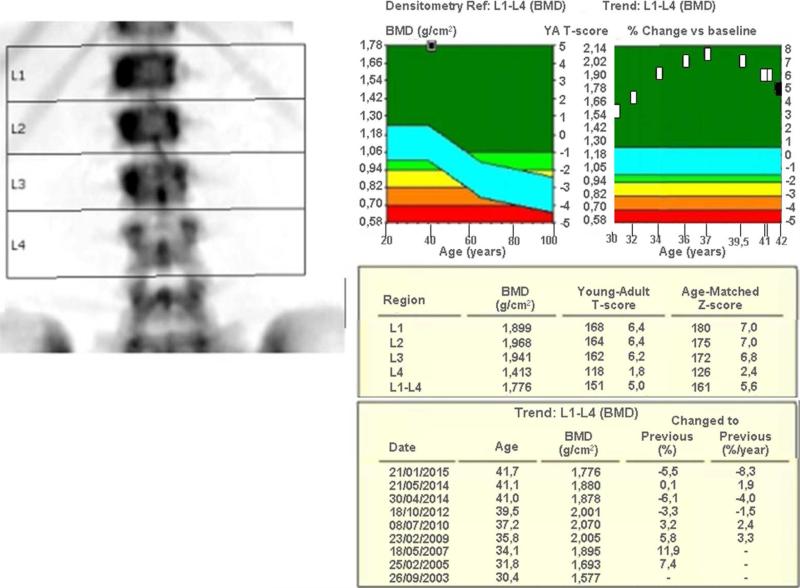
BMD studies were performed first using a dual photon absorptiometer (DP3 analyzer; Lunar) and then using DXA (Lunar Prodigy scanners) at the Hospital Clinic Barcelona.
We report a middle-aged woman with systemic lupus erythematosus (SLE) and idiopathic acquired osteosclerosis, involving diffusely her axial skeleton and focally her appendicular skeleton, that progressed over at least one decade but now is decreasing.
III) Material and Methods
Informed written consent for the research studies was obtained from the patient according to the Ethics Committee of the Hospital Clinic of Barcelona.
A) Case Report
This 42-year-old premenopausal Spanish woman had been referred to the Department of Rheumatology, Hospital Clinic, Barcelona in 1990 when she was 17 years-of-age for SLE and low bone mineral density (BMD). At that time, lumbar spine BMD assessed by dual photon absorptiometry was 79% the average value for age-matched controls while she was receiving glucocorticoid treatment.
SLE was diagnosed at 12 years-of-age in the context of fatigue, malar rash and photosensitivity, episodes of fever and arthralgias, and positive antinuclear and double-stranded DNA antibodies, as well as low serum complement levels.
At 20 years-of-age, kidney biopsy revealed lupus nephritis type III. She received hydroxychloroquine, azathioprine, and low-dose glucocorticoids. Five years later, worsening renal function with serum creatinine 1.6 mg/dl (Nl: 0.3-1.3 mg/dl) and re-biopsy led to intravenous cyclophosphamide therapy given monthly for eight months. The SLE became controlled and renal function stabilized while she received low-dose glucocorticoids, azathioprine, hydroxychloroquine, lisinopril, and saccharose-iron intravenously for periodic anaemia.
At age 30 years in 2003, her first DXA study (Lunar Prodigy, Madison, WI, USA) showed a lumbar spine BMD z-score of +3.8, and femoral neck and total hip BMD z-scores of −1.4 and −1.1, respectively (Figure 1 and Table 1). She had never fractured. Dietary calcium intake was no more than 500 mg/day, and she drank 1.5 liters of bottled water of low mineral content each day. She gave no history of intravenous drug abuse, tattoos, pierced ears, or blood transfusions. Menstrual cycles were regular since age 15 years. She had aches and pains but was otherwise healthy, worked as a laboratory technician without exposure to strontium or heavy metals, and raised her only child born in 2004. She had never received bone antiresorptive therapy. She took 4 mg of methylprednisolone daily from 2000 to 2012, and 2 mg per day since 2012. Her mother said that her daughter's dental history was uneventful during early childhood. There had been no loss of somewhat discolored adult teeth. Uric acid levels measured yearly since 1995 ranged from 2.9 to 6.4 mg/dL (Nl, 1.9 – 7.4). Family history was negative for diseases that cause osteosclerosis. DXA showed her father's T-score was −1.8 at the lumbar spine, sister's z-score −2.1 at the total hip, and mother's T-score −3.1 at the lumbar spine. Her brother was not studied.
Figure 1.
DXA (Lunar Prodigy, Madison, WI, USA) shows a marked and progressive increase in lumbar spine BMD from ages 30 to 37 years, followed by a steady decrease.
At age 42 years, physical examination showed a thin woman (height 157 cm; weight 46 Kg) without skeletal deformities. Her palate and mandible were unremarkable. There was no tenderness of the skull, ribs, pelvis, or spine. A dermatologist found no signs of mastocytosis.
B) Radiological Studies
In 1990, her lumbar spine BMD was measured by dual photon absorptiometry utilizing a DP3 instrument (Lunar, Madison, WI, USA). From 2003 to 2015, BMD was evaluated in the lumbar spine and proximal femur (neck and total hip) by DXA (Lunar Prodigy, Madison, WI, USA).
Radiographic skeletal survey at age 37 years in 2010 included anteroposterior (AP) and lateral images of the skull, thoracic and lumbar spine, and legs, together with AP views of the pelvis, arms, hands, and femurs. Whole-body bone scintigraphy and whole-body magnetic resonance imaging (MRI) and ultrasonography of the abdomen were performed at ages 34 and 37 years, respectively.
C) Laboratory Studies
Bone turnover markers (BTMs), assayed at the Biochemistry Department of Hospital Clinic, Barcelona using fasting blood and second-void urine samples collected between 8 and 10 a.m., comprised: serum bone-specific ALP by ELISA (Immunodiagnostic Systems, Boldon, England), C-terminal crosslinking telopeptide of type I collagen (CTX) and N-propeptide of procollagen type I (PINP) by electrochemiluminescence automated immunoassays (Elecsys, Roche), and osteocalcin (OC) by IRMA (Cis Bio, Sorgues, France), and urinary N-terminal crosslinking telopeptide of type I collagen (NTX) by ELISA (Osteomark® NTX, Alegre, Scarborough, ME, USA). Serum sclerostin (SOST) was quantited by ELISA (Biomedica, Wien, Austria) and plasma fibroblast growth factor-23 (FGF-23) by enzyme immunoassay (Immunotopics, San Clemente, CA, USA). Reference values were from our study of 164 healthy premenopausal Spanish women aged 35-45 years.(8) SOST reference values were from a similar group of 22 women.(9) To exclude specific osteosclerotic disorders, additional laboratory tests were performed (see Results).
D) Serum Multiplex Biomarker Profiling
Serum Multiplex Biomarker Profiling (SMBP) was carried out in 2013 in one comprehensive “batch” assay at Amgen, Inc. (Thousand Oaks, CA, USA) using our published methodology (Supplementary Appendix, Methods and Table 2)(10) including patient fasting serum from 2011 shipped frozen to Amgen, Inc. and from 8 healthy women 33 to 45 years-of-age ascertained in St. Louis in 2012.
Table 2.
Laboratory Findings
| Parameter | Normal Range | Result According to Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | 1990 | 1991 | 2003 | 2005 | 2007 | 2009 | 2010 | 2011 | 2012 | 2014 | 2015 | |
| Creatinine (mg/dL) | 0.30 – 1.30 | - | - | 1.3 | 1.2 | 1.24 | 1.18 | 1.32 | 1.38 | 1.37 | 1.52 | 1.65 |
| Glomerular filtrate (eGFR) | > 60 | 111 | 97 | 52 | - | 53 | 55 | 48 | 46 | 46 | 40 | 38 |
| CRP (mg/dL) | < 1.00 | - | - | 0.5 | 0.9 | 0.2 | 0.03 | 0.05 | 0.1 | 0.05 | 0.73 | 0.03 |
| ESR (mm/h) | < 20 | - | - | - | 19 | 7 | 10 | 11 | 9 | 11 | 10 | 12 |
| Complement C3 (g/L) | 0.870 – 1.700 | - | - | 0.826 | 0.741 | 0.853 | 0.808 | 0.781 | 0.893 | 0.791 | 0.815 | 0.790 |
| Ferritin (ng/mL) | 15 – 200 | - | - | 52 | 28 | 41 | 28 | 44 | 9 | 14 | 54 | 41 |
| Transferrin (g/L) | 2.5 – 3.8 | - | - | - | 2.74 | - | - | - | 3 | 3.2 | 2.4 | 2.4 |
| Transferrin saturation (%) | 16 – 45 | - | - | - | 23 | - | - | - | 4.23 | 11.6 | 13.6 | 14.7 |
| Serum Iron (mcg/dL) | 50 – 170 | 69 | 102 | 92 | - | 66 | 72 | 73 | 37 | 53 | 46 | 50 |
| Leukocytes count (×109) | 4.000 – 11.000 | 7.500 | 6.000 | 9.000 | 6.730 | 8.480 | 5.290 | 9.130 | 6.140 | 6.400 | ||
| Hemoglobin (g/dL) | 12 – 17 | 12.5 | 12.1 | 11.6 | 12.5 | 12.8 | 10.7 | 12.9 | 13.2 | 13.5 | ||
| Hematocrit (%) | 36 – 41 | 39 | 38 | 35 | 39 | 40 | 36 | 41 | 42 | 43 | ||
| Platelets count (×109) | 120 – 400 | 266 | 303 | 340 | 290 | 266 | 342 | 302 | 249 | 272 | ||
| Calcium (mg/dL) | 8.5 – 10.5 | - | - | 10.3 | 8.8 | 9.8 | 9.9 | 9.2 | 9.3 | 9 | 9.4 | 9.4 |
| Phosphate (mg/dL) | 2.3 – 4.3 | - | - | 2.8 | 3.2 | 3.9 | 3.4 | 4.2 | 4.3 | 3.4 | 3.5 | 4 |
| Total ALP (U/L) | 80 – 250 | - | - | 297 | 211 | 200 | 207 | 192 | 176 | 204 | 186 | 89** |
| Bone ALP (ng/mL) | 6 – 13.6 | 23 | 19.7 | 19 | 30 | 22.4 | - | 23.9 | 20.8 | - | ||
| P1NP (ng/mL) | 23 – 63 | - | - | - | 126 | 178 | 156 | 169 | - | 133 | 116 | - |
| OC (ng/mL) | 8 – 23 | - | - | - | 31 | 52 | - | 52 | - | 45 | 54 | - |
| CTX (ng/mL) | 0.14 – 0.48 | - | - | - | - | 0.68 | 0.92 | 0.73 | - | 0.68 | 0.62 | - |
| PTH (pg/mL)* | 10 – 65 | - | - | 46 | 130 | 75 | 81 | 112 | - | 91 | 81 | 215 |
| 25-OH-D (ng/mL) | ≥ 30 | - | - | 31 | 25 | 14 | 13 | 12.4 | - | 13.2 | 11.6 | 13.7 |
| 1,25-OH-D (pg/mL)* | 18 - 70 | - | - | 37 | 30 | 19 | 37 | 48 | - | 29 | - | - |
| FGF-23 (RU/mL)* | < 130 | - | - | - | - | - | - | 180 | - | - | - | - |
| SOST (pmol/L) | 42.7 ± 10 | - | - | - | - | - | - | 110 147 |
- | - | - | - |
| Urine | ||||||||||||
| Calcium (mg/24h) | 50 – 250 | 57 | 146 | 17 | - | 18 | - | 30 | 29 | 16 | 20 | - |
| NTX (ng/mL) | 20 – 69 | - | - | - | 98 | 88.7 | 113 | 86 | - | 104 | 113 | - |
plasma
New lab method for total ALP detection (46-116)
ALP: Alkaline phosphatase; PINP: N-propeptide of procollagen type I; OC: osteocalcin; CTX: C-terminal crosslinking telopeptide of type I collagen; NTX: N-terminal crosslinking telopeptide of type I collagen; PTH: Parathyroid hormone; 25-OH-D: 25- hydroxyvitamin D; 1,25-OH-D: 1,25-dihydroxyvitamin D; FGF-23: fibroblast growth factor-23; SOST: sclerostin.
E) Bone Marrow and Transiliac Bone Biopsy
When the patient was 38-years-old, an iliac crest specimen was obtained using a 7 mm inner diameter Bordier trephine and fixed in 70% ethanol, dehydrated, embedded in methylmethacrylate, and sectioned for non-decalcified histologic examination including von Kossa staining. Oxytetracycline, 250 mg thrice daily for three days, had been given twice before biopsy, with an interval of 11 days. Additional iliac crest specimens were decalcified, embedded in paraffin, and stained with Hematoxylin and Eosin. A bone marrow biopsy was also obtained during the procedure.
F) Mutation Analyses
Sanger sequencing of LRP5 (exons 2, 3, and 4) and LRP4 (exons 25 and 26), where high bone mass-associated mutations are found, as well as SOST (all coding exons and adjacent mRNA splice sites) was performed in our research laboratory in St. Louis, MO, USA. We also performed NGS sequencing using the Ion Torrent platform (Applied Biosystems, Carlsbad, CA) for 35 genes involved in bone turnover or function including all osteopetrosis / high bone mass genes. Copy number microarray (Cytoscan HD, Affymetrix, Santa Clara, CA) was performed at the GTAC core lab, Washington University School of Medicine, St Louis, MO.
IV) Results
A) Radiological Findings
DXA beginning in 2003 and spanning patient ages 30 to 42 years showed lumbar spine z-scores that steadily increased from +3.8 and peaked at +7.9 in 2010. During this time, femoral neck and total hip z-scores increased from −1.4 to −0.7 and from −1.1 to +0.6, respectively (Table 1 and Figure 1). Then, beginning in 2012 without changes in her lifestyle or diet, the z-scores decreased steadily. During 2012 - 2015, the only changes in her treatment had been an azathioprine dose lowered from 50 to 25 mg daily, prednisolone lowered from 4 to 2 mg daily, iron administrated intravenously only once or twice yearly, and introduction of a progestogen-containing intrauterine device.
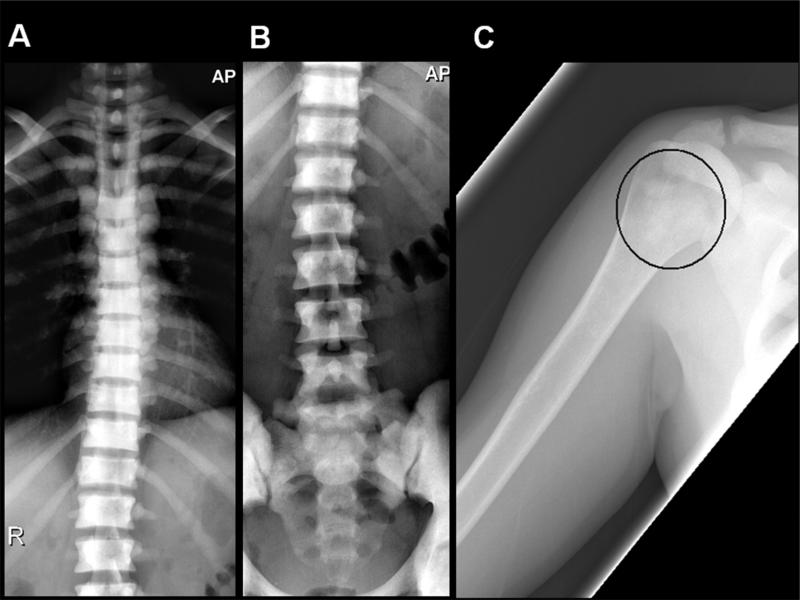
The radiographic skeletal survey at age 37 years in 2010 demonstrated diffuse osteosclerosis of the axial skeleton (ribs, thoracic and lumbar spine, and pelvis) (Figure 2A,B) with a normal skull. Patchy geographic well-marginated areas of intramedullary sclerosis of a different character were in the proximal humeri (Figure 2C) and femurs. The appendicular skeleton was otherwise unaffected. There was no ligamentous calcification or ossification, or trabecular coarsening to suggest skeletal fluorosis or other halide poisoning. No bone enlargement or cortical and trabecular thickening was present to indicate Paget's bone disease. Skeletal metastasis was unlikely given the many years of follow-up without manifestations from a primary source. No findings suggested renal osteodystrophy, which seemed unlikely based on the laboratory findings.
Figure 2.
Anteroposterior radiographs of the thoracic and lumbar spine (A,B) demonstrate severe diffuse osteosclerosis of the vertebral bodies with loss of normal cortical-medullary differentiation. Imaged portions of the ribs and pelvis shows similar findings. Anteroposterior radiograph of the right proximal humerus (C) demonstrates patchy intramedullary sclerosis of the proximal shaft (ellipse), different in character from the diffuse marrow replacement of the axial skeleton.
Whole-body bone scintigraphy at age 34 years in 2007 showed increased radionuclide uptake in several thoracic vertebral bodies, and more focally in the right sacrum (Supplementary Appendix, Figure 1). Diffuse uptake throughout marrow-rich areas of the axial skeleton was not present.
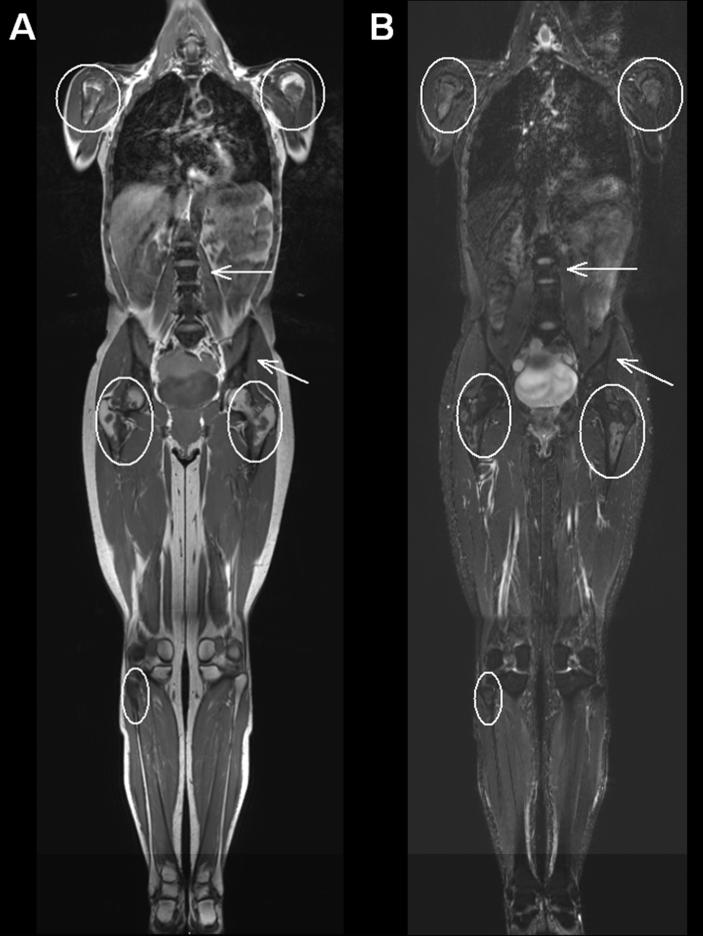
Whole-body MRI at age 37 years showed two patterns of marrow involvement: 1) diffuse, severe, T1- and STIR-hypointense marrow in the cervical, thoracic, and lumbar spine, ribs, scapulae, and pelvis, which could represent marrow deposition disease such as hemosiderosis, marrow replacement as in myelofibrosis, mastocytosis or Gaucher disease, diffuse osteoblastic metastasis, or metabolic bone sclerosis; and 2) patchy areas of marrow replacement in the proximal and midshafts of the long bones, especially the humeri and femurs, with relative sparing of the epiphyses and apophyses. The signal intensity of these areas was hyperintense to skeletal muscle and hypointense to subcutaneous fat on T1-weighted images, and intermediate on STIR sequences, and was most consistent with red marrow reconversion (Figure 3). There were a few isolated foci within the long bones that appeared to have central fatty marrow signal intensity, suggesting the possibility of underlying bone infarcts which would correlate with the patchy intramedullary sclerosis of the proximal long bones on radiographs.
Figure 3. Whole body MRI.
Coronal T1-weighted (A) and STIR (B) whole-body MR images demonstrate severe signal hypointensity of the scapulae, ribs, spine and pelvis, which could represent marrow deposition disease such as hemosiderosis, marrow replacement as in myelofibrosis, mastocytosis or Gaucher disease, diffuse osteoblastic metastasis, or metabolic bone sclerosis (arrows). Within the proximal long bones, there is T1-isointense and hypointense signal compared to skeletal muscle and intermediate STIR signal sparing the apophyses and epiphyses (ellipses), most consistent with red marrow reconversion.
Ultrasonography of the abdomen showed slight hepatomegaly and normal spleen size (6.5 cm). There were no renal alterations.
Overall, the skeletal imaging findings were most consistent with a marrow-replacing process.
B) Laboratory Findings
Table 2 summarizes the laboratory findings. Serum calcium and phosphorus levels were always normal despite 25OHD of < 20 ng/mL (Nl > 30 ng/mL) and PTH of 46 – 130 pg/mL (Nl: 10 65 pg/mL). Hypocalciuria of 16 to 30 mg calcium/24 h was present while the eGFR was 38 – 55 mls/min. Serum iron levels were low or normal (between 37 – 92 mcg/dL) as were ferritin levels and transferrin saturation. Peripheral blood leukocyte count was normal with isolated schistocytes.
BTMs assayed in Barcelona were generally 1.6- to 2.8-fold above our upper limits of normal (ULN). Those reflecting formation (bone ALP, PINP, and OC) were increased 2.2-, 2.8-, and 2.3-fold, respectively, whereas those reflecting resorption (CTX and NTX) were increased 1.9- and 1.6-fold, respectively. Plasma FGF-23 was slightly increased. Notably, serum SOST assayed twice in 2010 was 2.5 and 3.5 fold above the ULN (Table 2), and verified by SMBP (see below). Similar findings were noted for RANKL.
C) Serum Multiplex Biomarker Profiling
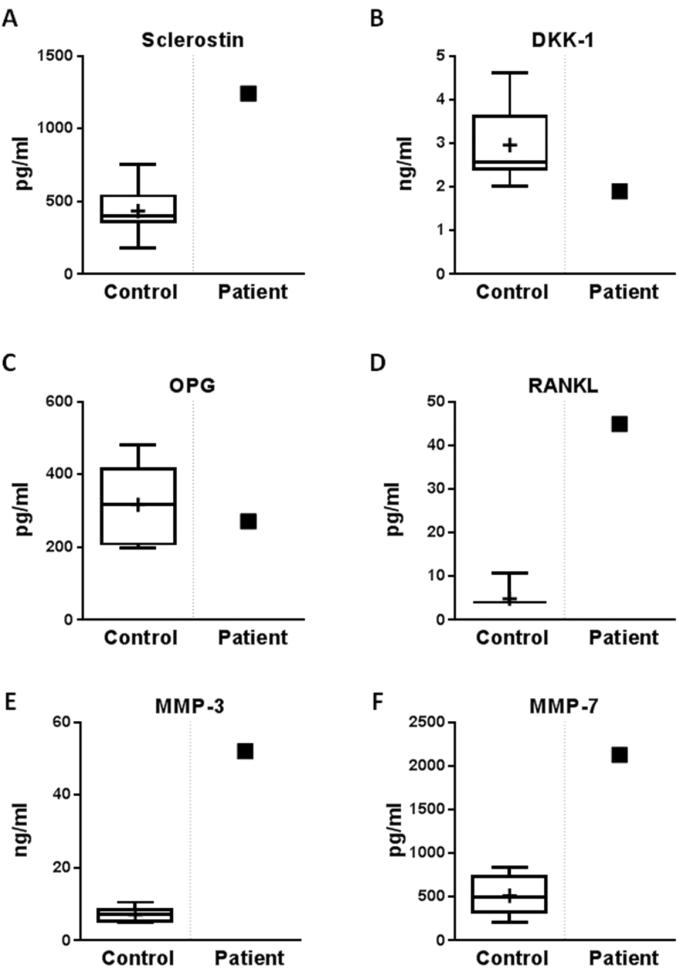
SMBP findings (Figure 4, Supplementary Figure 2 and Table 3) included elevated matrix metalloproteinase (MMP)-3 and −7 levels, whereas MMP-1 and MMP-8 levels were normal. SMBP verified the high SOST and RANKL levels and, of interest, revealed a slightly low DKK-1 level.
Figure 4. Serum multiplex biomarker profiling.
Serum levels of sclerostin (A), DKK-1 (B), OPG (C), RANKL (D), matrix metalloproteinases MMP-3 (E), and MMP-7 (F) (arrows) are contrasted with normal control values plotted by age and gender.
Table 3.
Additional Laboratory Testing For Osteosclerotic Disorders
| Parameter | Normal Range | Value |
|---|---|---|
| Serum | ||
| Fluoride (μg/L) | 10 – 50 | 33 |
| Retinol (μg/L) | 30 – 70 | 69 |
| TSH (mIU/L) | 0.4 – 4 | 0.75 |
| Tryptase (ng/mL) | 0 – 13 | 10.2 |
| Heavy metals | ||
| Al, Cd, Cr, Ni, Pb, Mn | - | Normal |
| Protein electrophoresis | - | Normal |
| Serology | ||
| HbsAg, HbsAb, HbcAb, VHC, HIV | - | Negative |
| Urine | ||
| Fluoride (μg/L) | 156 – 1800 | 280 |
D) Additional Laboratory Tests
Tests for osteosclerotic disorders included normal serum and urine electrophoresis and fluoride levels and normal serum retinol and tryptase levels and negative serologic tests for hepatitis C virus and HIV infection (Table 3). Any sickle cell trait was excluded by peripheral blood smear and normal hemoglobin electrophoresis. Angiotensin converting enzyme (ACE) assay to screen for sarcoidosis was not performed.
E) Bone Marrow and Transiliac Crest Biopsy
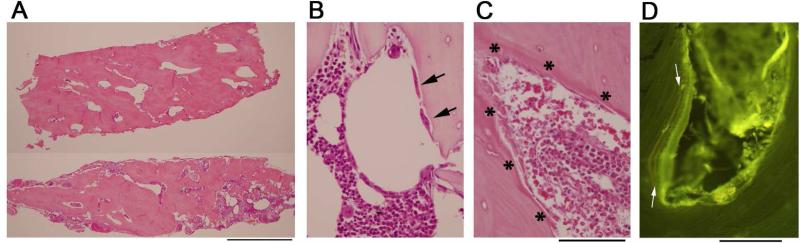
Decalcified iliac crest specimens showed dense lamellar bone with a lack of distinction between cortex and spongiosa (Figure 5A). All three hematopoietic lineages were represented, and osteoclasts and osteoblasts were not increased or morphologically abnormal (Figure 5B, C). There was no peritrabecular fibrosis, and no granulomas. Mast cells were not increased, as assessed by CD117 immunostains (not shown).
Figure 5. Iliac crest biopsies.
Hematoxylin and eosin stained sections of the decalcified iliac crest biopsy specimens show increased and thickened trabeculae that are not well demarcated from cortical bone (A). The limited amounts of marrow present show trilineage hematopoiesis (B,C). A few morphologically unremarkable osteoclasts are present (B, arrows). Osteoblasts are also present with normal morphology (C, asterix). Examination of the nondecalcified biopsy under fluorescence demonstrates well-defined double tetracycline labels, here seen under a layer of osteoid (D, yellow bands between arrows). Scale bars (A) 2 mm; (B,C) 100 μm; (D) 100 μm.
Undecalcified specimens showed similar changes, with thick trabeculae. Von Kossa staining did not indicate an increase in osteoid (not shown). Fluorescence microscopy of an unstained section demonstrated distinct double labels of tetracycline indicative of a normal rate of mineralization (Figure 5D).
F) Mutation Analyses
By Sanger sequencing, no mutations were detected in the genes that encode LRP5 (exons 2, 3, and 4), LRP4 (exons 25 and 26), or SOST. NGS sequencing, including known genes involved in osteopetrosis and high bone mass phenotypes (TNFRSF11A, TNFSF11, CA2, CLCN7, CTSK, OSTM1, PlEKM1, TCIRG1, SOST, SLC29A3, LRP4, LRP5, LRP6, SNX10, FAM20C, FAM123B, TYROBP), was unremarkable. Whole-genome microarray showed no notable deviations in normal copy number (2), including the region downstream of SOST, where a 52 kb deletion was reported in patients with van Buchem disease.(11)
V) Discussion
Acquired, widely distributed osteosclerosis affected our middle-aged patient with SLE diagnosed during adolescence. This skeletal aberration was first detected at 30 years-of-age using DXA. At age 37 years, radiographic survey revealed diffuse osteosclerosis in the central skeleton with focal sclerosis in proximal areas of long bones. Nevertheless, extensive evaluation disclosed no condition that causes generalized or focal increases in skeletal mass. Her family's negative medical history and DXA results, as well as analyses for some mutations associated with high bone mass, gave no evidence of an environmental or genetic basis for this finding.(12) Iliac crest histopathology showed a large amount of normal-appearing lamellar bone with unremarkable osteoblasts and osteoclasts, and an absence of tumor, peritrabecular fibrosis, or mast cell granulomas.(13) Remarkably, her osteosclerosis progressed for at least 11 years, but now seems to be resolving.
We considered a diagnosis of autoimmune myelofibrosis,(14) however this was excluded. Although her positive antinuclear antibodies, absence of splenomegaly, and reticulin fibrosis in some areas of the bone marrow specimen favored this possibility, against this diagnosis was her absence of an SLE flare during the last few years,(15-16) no cytopenia or major peripheral blood abnormalities, negative Coombs test, and marrow with the three hematopoietic precursors, normal cellularity, and absence of lymphoid infiltration.(14-16) Additionally, her osteosclerosis had diminished recently without specific treatment such as glucocorticoids at high doses.
We also considered whether the osteosclerosis was drug-induced, particularly when her BMD decreased after the dose of azathioprine and IV infusions of iron were diminished. However, to our knowledge, azathioprine has not been linked with metabolic bone diseases, including osteosclerosis, although rats given azathioprine acquire inhibited bone formation and mineralization.(17) Saccharose iron administered intravenously has caused FGF-23-mediated hypophosphatemic osteomalacia,(18-19) but our patient's plasma FGF-23 level was normal or only slightly increased when her eGFR was 48 mls/min, urinary excretion of phosphorus was consistently normal, and bone formation was active on iliac crest biopsy. BTMs usually showed elevated values (formation higher than resorption), but were normal in the one evaluation by SMBP. We do not know if the elevated BTMs could be partly attributed to her elevated bone mass per se. We and others have reported a similar increase in BTMs in Camurati-Engelmann disease, a sclerosing bone dysplasia featuring rapid bone turnover in which BTM levels correlate with disease activity assessed by bone scintigraphy.(20-21) Our patient's BMT levels seemed consistent with her sequential DXA findings.
A striking and consistent finding was her elevated serum SOST and RANKL levels. This seemed associated with a slightly decreased level of another Wnt-signaling antagonist, DKK-1. Although we cannot exclude a compensatory lowering of circulating DKK1 due to her elevated SOST,(9,22) previous data suggest independent effects of these Wnt-antagonists.(23) We did consider whether her elevated BTMs and especially SOST levels reflected her compromised renal function.(24) Circulating SOST is increased in chronic kidney disease, with levels about 2-3 fold elevated and paralleling the rise in PTH and FGF-23 before kidney transplantation.(25) However, her eGFR between 38 and 55 ml/min through 2003 and 2015 was not sufficiently impaired to account for her hypersclerostinemia. SOST is secreted predominantly by osteocytes and inhibits bone formation by osteoblasts.(26) In two sclerosing bone dysplasias, sclerosteosis and van Buchem disease, there is genetically-based SOST deficiency causing excessive osteoblast activity.(26-28) Hence, our patient's hypersclerostinemia could seem paradoxical. In the high-bone-mass disorder due to activating mutations within LRP5 causing increased WNT signalling,(29) circulating SOST levels are normal(29-30) or increased.(31) For our patient, mutation analysis was negative for SOST, LRP5, LRP4, and osteopetrosis genes. There was no deviation in normal copy number near the SOST gene, so our patient did not have a deletion similar to the 52 kb deletion reported in van Buchem disease patients. The cause of her hypersclerostinemia is unknown, but could represent an unsuccessful compensatory effect for her high bone mass. Alternatively, peripheral resistance to SOST with accelerated bone remodeling including exuberant bone acquisition could be causing her osteosclerosis(32) and account for her hypersclerostinemia, while circulating DKK-1 is in turn low. However, others have reported no association between total-body bone mineral content and serum SOST levels between ages 20 to 39 years.(33) Of interest, Tsui et al(34) recently described in ankylosing spondylitis a relationship between autoimmunity and neo-ossification, perhaps mediated by IgG autoantibodies to SOST leading to reduced functional SOST. Unfortunately, we were unable to test for autoantibodies to SOST, DKK-1, or other components of WNT signalling. Additionally, her serum RANKL levels were elevated, suggesting that her high bone mass perhaps also reflected a block in RANKL action. In fact, in 2009, Riches et al.(35) reported high-turnover osteoporosis associated with anti-osteoprotegerin antibodies in patients with celiac disease.
SMBP also revealed high circulating levels of Cathepsin K, matrix metalloproteinases MMP-3 and MMP-7 in our patient. This could reflect her high bone remodelling rate, particularly concerning resorption. Bone cells express a number of MMPs(36) that act in remodelling and repair. MMP-3 degrades collagen types II, III, IV, IX, and X, proteoglycans, fibronectin, laminin, and elastin,(37) and can activate other MMPs such as MMP-1, MMP-7, and MMP-9, rendering MMP-3 crucial in connective tissue remodelling.(38) Additionally, MMP-3 and MMP-9 are up-regulated in osteoblasts in sclerotic compared to non-sclerotic bone taken from subchondral bone under mechanical cyclic compression.(39) Nevertheless, serum MMP levels have not been quantitated in diffuse osteosclerosis.
Because our patient has SLE, perhaps her osteosclerosis has an autoimmune basis. However, there are no reports of increased antagonists to, or activation of, Wnt-signaling in SLE to support this possibility. Furthermore, we do not know of reports of anti-RANKL antibodies in SLE. Her osteosclerosis appears predominantly axial and not generalized. Thus, its etiology remains unknown, but her elevated circulating SOST and RANKL levels may reflect skeletal resistance to these key regulators of bone turnover. Interestingly, antibodies against SOST (blosozumab and romosozumab) and RANKL (denosumab) have been investigated as treatments for osteoporosis.(40-42)
Supplementary Material
VI) Acknowledgments
We are grateful to Ms. Margaret Huskey and Ms. Shenghui Duan for assisting with the gene sequencing studies. Dr. Fan Zhang illustrated the serum multiplex biomarker profiling. Dr. Luis del Rio and Dr. Africa Muxi helped review the bone densitometry data. Dr Jaume Pomés contributed to the radiological work. Dr. Maria Rozman interpreted the bone marrow biopsy and contributed the illustrations. Dr. Carme Mallofré helped interpret the transiliac bone biopsy findings.
Supported by Shriners Hospitals for Children, The Clark and Mildred Cox Inherited Metabolic Bone Disease Research Fund at the Barnes-Jewish Hospital Foundation, The Frederick S. Upton Foundation, and the National Institute of Diabetes and Digestive and Kidney Diseases (DK067145). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented in part at the 36th Annual Meeting of the American Society for Bone and Mineral Research (September 12-15, 2014, Houston, TX, USA) [J Bone Miner Res 29 (Suppl 1). Available at http://www.asbmr.org/education/AbstractDetail?aid=8c7bc2b3-c05a-4c55-82ca-f1da8907ca73.]
Disclosures: None.
Authors’ roles:
All authors helped write and approved the submitted manuscript. NG diagnosed and investigated the patient's osteosclerosis. LG and SRG contributed to the clinical assessments and data collection. JD and DVN characterized, illustrated, and discussed the radiological and histopathological findings, respectively. MS performed and helped interpret the serum multiplex biomarker profiling. SM performed and interpreted the mutation analyses. MPW helped guide the patient studies and manuscript creation.
References
- 1.Whyte MP. Primer On Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8th Ed Vol. 85. American Society for Bone and Mineral Research; Wiley Blackwell: 2013. Sclerosing Bone Disorders. p. 769. [Google Scholar]
- 2.Gregson CL, Hardcastle SA, Cooper C, Tobias JH. Friend or foe: high bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology (Oxford) 2013;52:968–85. doi: 10.1093/rheumatology/ket007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whyte MP, Madson KL, Mumm S, et al. Rapid skeletal turnover in a radiographic mimic of osteopetrosis. J Bone Miner Res. 2014;29:2601–9. doi: 10.1002/jbmr.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ustün N, Ustün I, Ozgür T, et al. Diffuse osteosclerosis in a patient with prostate cancer. Osteoporos Int. 2014;25:1181–5. doi: 10.1007/s00198-013-2545-9. [DOI] [PubMed] [Google Scholar]
- 5.Ihde LL, Forrester DM, Gottsegen CJ, et al. Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31:1865–82. doi: 10.1148/rg.317115093. [DOI] [PubMed] [Google Scholar]
- 6.Resnick D, Kransdorf MJ. Chapter 72: Skeletal metastases. In: Resnick D, Kransdorf MJ, editors. Bone and Joint Imaging. 3rd ed. Elsevier; Philadelphia, PA: 2005. [Google Scholar]
- 7.Lacy MQ, Gertz MA, Hanson CA, Inwards DJ, Kyle RA. Multiple myeloma associated with diffuse osteosclerotic bone lesions: a clinical entity distinct from osteosclerotic myeloma (POEMS syndrome). Review. Am J Hematol. 1997;56:288–93. doi: 10.1002/(sici)1096-8652(199712)56:4<288::aid-ajh16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Guañabens N, Filella X, Monegal A, et al. Reference intervals for bone turnover markers in Spanish premenopausal women. Clin Chem Lab Med. 2016;54:293–303. doi: 10.1515/cclm-2015-0162. [DOI] [PubMed] [Google Scholar]
- 9.Gifre L, Ruiz Gaspà S, Monegal A, et al. Effect of glucocorticoids treatment on Wnt signalling antagonists (sclerostin and Dkk 1) and their relationship with bone turnover. Bone. 2013;57:272–6. doi: 10.1016/j.bone.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Whyte MP, Wenkert D, Dwyer DC, Lacey DL, Stolina M. Systemic biomarker profiling of metabolic and dysplastic skeletal diseases using multiplex serum protein analyses. (Abstract) J Bone Miner Res. 2010;25(Suppl 1):S137. [Google Scholar]
- 11.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL receptor related protein 5. N Engl J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 13.Fallon MD, Whyte MP, Teitelbaum SL. Systemic mastocytosis associated with generalized osteopenia: histological characterization of the skeletal lesion using undecalcified bone from two patients. Human Pathology. 1981;12:813–20. doi: 10.1016/s0046-8177(81)80084-6. [DOI] [PubMed] [Google Scholar]
- 14.Vergara Lluri ME, Piatek CI, Pullarkat V, et al. Autoimmune myelofibrosis: an update on morphologic features in 29 cases and review of the literature. Human Pathology. 2014;45:2183–91. doi: 10.1016/j.humpath.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Pundole X, Konoplev S, Hlaing Oo T, Lu H. Autoimmune myelofibrosis and systemic lupus erythematosus in a middle aged male presenting only with severe anemia: A case report. Medicine (Baltimore) 2015;94(19):1–4. doi: 10.1097/MD.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquette RL, Meshkinpour A, Rosen PJ. Autoimmune myelofibrosis. A steroid responsive cause of bone marrow fibrosis associated with systemic lupus erythematosus. Medicine (Baltimore) 1994;73(3):145–52. [PubMed] [Google Scholar]
- 17.Cegiela U, Kaczmarczyk Sedlak I, Pytlik M, Folwarczna J, Nowinska B, Fronczek Sokól J. Alendronate prevents development of the skeletal changes induced by azathioprine in rats. Acta Pol Pharm Drug Res. 2013;70:309–15. [PubMed] [Google Scholar]
- 18.Yamamoto S, Okada Y, Mori H, Kurozumi A, Torimoto K, Arao T, et al. Iatrogenic osteomalacia: report of two cases. J UOEH. 2013;35:25–31. doi: 10.7888/juoeh.35.25. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto S, Okada Y, Mori H, Fukumoto S, Tanaka Y. Fibroblast growth factor 23 related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern Med. 2012;51:2375–8. doi: 10.2169/internalmedicine.51.7450. [DOI] [PubMed] [Google Scholar]
- 20.Whyte MP, Totty WG, Novack DV, Zhang X, Wenkert D, Mumm S. Camurati Engelmann disease: unique variant featuring a novel mutation in TGFβ1 encoding transforming growth factor beta 1 and a missense change in TNFSF11 encoding RANK ligand. J Bone Miner Res. 2011;26:920–33. doi: 10.1002/jbmr.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernández MV, Peris P, Guañabens N, et al. Biochemical markers of bone turnover in Camurati Engelmann disease: a report on four cases in one family. Calcif Tissue Int. 1997;61:48–51. doi: 10.1007/s002239900293. [DOI] [PubMed] [Google Scholar]
- 22.Van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE. Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab. 2014;99:E252–6. doi: 10.1210/jc.2013-3278. [DOI] [PubMed] [Google Scholar]
- 23.Balemans W, Piters E, Cleiren E, et al. The binding between sclerostin and LRP5 is altered by DKK1 and by high bone mass LRP5 mutations. Calcif Tissue Int. 2008;82:445–53. doi: 10.1007/s00223-008-9130-9. [DOI] [PubMed] [Google Scholar]
- 24.Thambiah S, Roplekar R, Manghat P, et al. Circulating Sclerostin and Dickkopf 1 (DKK1) in Predialysis Chronic Kidney Disease (CKD): Relationship with Bone Density and Arterial Stiffness. Calcif Tissue Int. 2012;90:473–80. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- 25.Bonani M, Rodriguez D, Fehr T, et al. Sclerostin Blood Levels Before and After Kidney Transplantation. Kidney Blood Press Res. 2014;39:230–9. doi: 10.1159/000355781. [DOI] [PubMed] [Google Scholar]
- 26.Bellido T. Osteocyte driven bone remodeling. Calcif Tissue Int. 2014;94:25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moester MJ, Papapoulos SE, Löwik CW, van Bezooijen RL. Sclerostin: Current knowledge and future perspectives. Calcif Tissue Int. 2010;87:99–107. doi: 10.1007/s00223-010-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Lierop A, Hamdy N, van Egmond M, Bakker E, Dikkers F, Papapoulos S. Van Buchem disease: clinical, biochemical and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28:848–854. doi: 10.1002/jbmr.1794. [DOI] [PubMed] [Google Scholar]
- 29.Pangrazio A, Boudin E, Piters E, et al. Identification of the first deletion in the LRP5 gene in a patient with autosomal dominant osteopetrosis type I. Bone. 2011;49:568–71. doi: 10.1016/j.bone.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregson CL, Poole KE, McCloskey EV, et al. Elevated circulating Sclerostin concentrations in individuals with high bone mass, with and without LRP5 mutations. J Clin Endocrinol Metab. 2014;99:2897–907. doi: 10.1210/jc.2013-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson CA, Foer D, Lee GS, et al. Serum levels of sclerostin, Dickkopf 1, and secreted frizzled related protein 4 are not changed in individuals with high bone mass causing mutations in LRP5. Osteoporos Int. 2014;25:2383–8. doi: 10.1007/s00198-014-2767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost M, Andersen T, Gossiel F, et al. Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high bone mass phenotype due to a mutation in Lrp5. J Bone Miner Res. 2011;26:1721–8. doi: 10.1002/jbmr.376. [DOI] [PubMed] [Google Scholar]
- 33.Mödder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–9. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsui FW, Tsui HW, Las Heras F, Pritzker KP, Inman RD. Serum levels of novel noggin and sclerostin immune complexes are elevated in ankylosing spondylitis. Ann Rheum Dis. 2014;73:1873–9. doi: 10.1136/annrheumdis-2013-203630. [DOI] [PubMed] [Google Scholar]
- 35.Riches PL, McRorie E, Fraser WD, Determann C, van't Hof R, Ralston SH. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med. 2009;361:1459–65. doi: 10.1056/NEJMoa0810925. [DOI] [PubMed] [Google Scholar]
- 36.Andersen TL, Ovejero MC, Kirkegaard T, Lenhard T, Foged NT, Delaissé JM. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004;35:1107–19. doi: 10.1016/j.bone.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Loffek S, Schilling O, Franzke CW. Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 38.Page McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez C, Pesesse L, Gabay O, et al. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 2012;64:1193–203. doi: 10.1002/art.33445. [DOI] [PubMed] [Google Scholar]
- 40.Fijalkowski I, Boudin E, Mortier G, Van Hul W. Sclerosing bone dysplasias: leads toward novel osteoporosis treatments. Curr Osteoporos Rep. 2014;12:243–51. doi: 10.1007/s11914-014-0220-5. [DOI] [PubMed] [Google Scholar]
- 41.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. New Engl J Med. 2014;370:412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 42.McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single and multiple dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29:935–43. doi: 10.1002/jbmr.2092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.