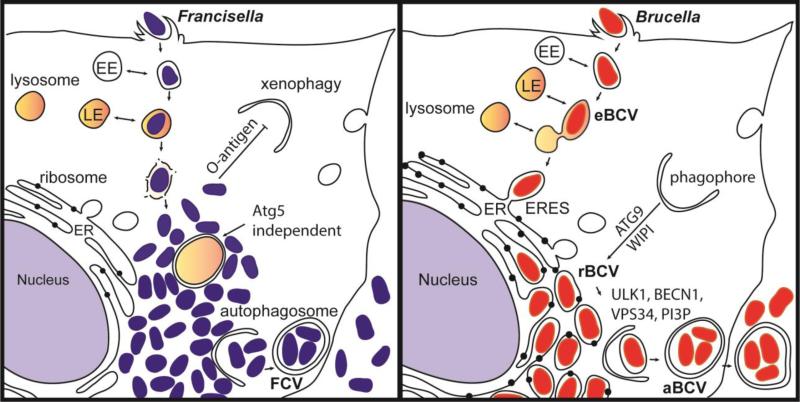
Figure 3. Intracellular niches of Francisella spp. and Brucella spp.
(A) Following internalization, Francisella resides in a vacuole that sequentially acquires markers of early endosomes (EE) and late endosomes (LE). Francisella escapes the original vacuole before lysosomal fusion to reach the cytosol. Once in the cytosol, the F. tularensis capsular and lipopolysaccharide O-antigen is an essential component of xenophagy avoidance. Francisella replicates rapidly in the cytosol of host cells and is found adjacent to autophagosomes. F. tularensis acquires amino acids generated via autophagy in an Atg5 independent manner. After extensive replication in murine macrophages, some bacteria are found in Francisella-containing vacuoles (FCVs). (B) Brucella exploits functions of the host secretory pathway to grow within membrane bound compartments called Brucella-containing vacuoles (BCV) throughout its intracellular life cycle. Brucella first traffics along the endocytic pathway in an endosomal BCV (eBCV), which acquires early endosomal (EE) markers then late endosomal (LE) markers, and partially fuses with lysosomes. Brucella then accesses the secretory pathway via interactions with endoplasmic reticulum (ER) exit sites (ERES) to access the ER. B. abortus requires the autophagy proteins ATG9 and WIPI, for biogenesis of the replicative BCV (rBCV) derived from ER membranes. After extensive replication, some rBCV are engulfed by autophagosome-like structures to become autophagic BCVs (aBCVs), which are involved in bacterial release and cell-to-cell spread. B. abortus selectively subverts several autophagy initiation complexes containing ULK1, BECN1, VPS34 and PI3P to generate an autophagic vacuole important for egress and cell-to-cell spread.