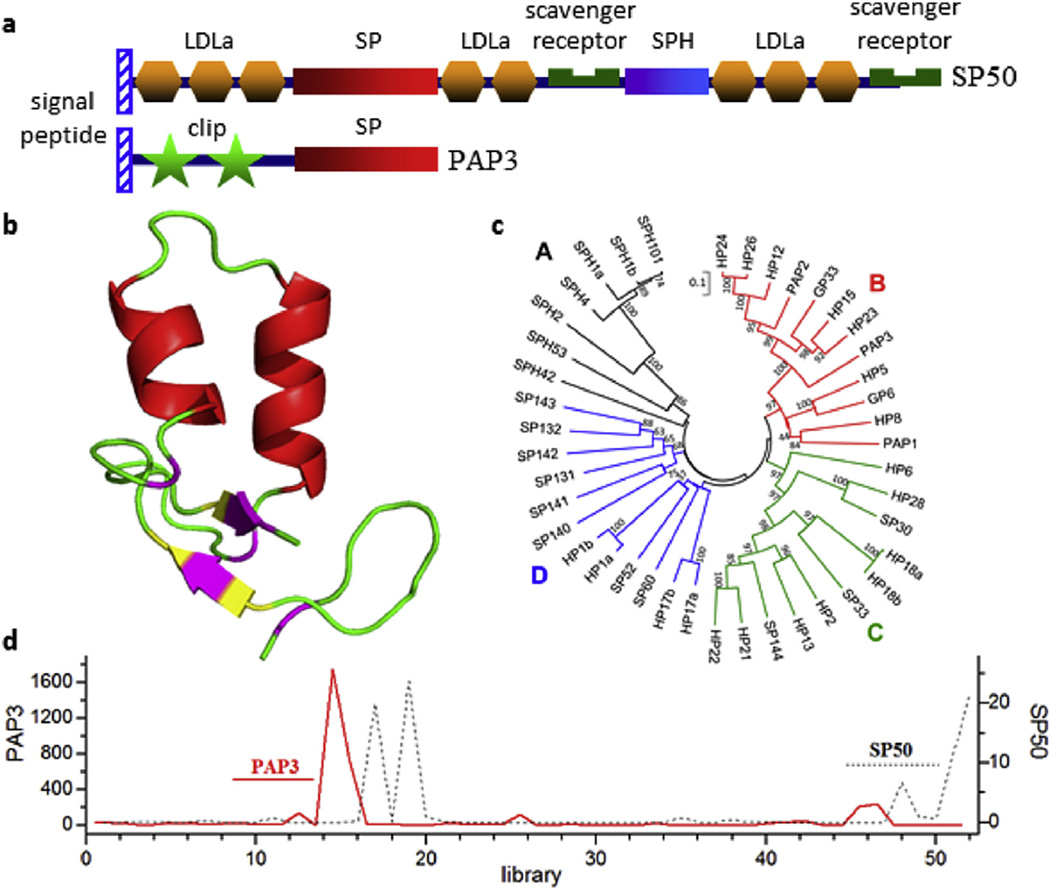
Fig. 10.
Domain architecture, structural model, phylogenetic relationships, and expression profiles for some of the nondigestive serine proteases and serine protease homologs (SPs/ SPHs) in M. sexta. a) SP50, representing the 52 multidomain SPs/SPHs, has the domains organized in the same way as those in its ortholog Drosophila Nudel; PAP3, one of the 42 clipdomain SPs/SPHs, activates proPOs in the presence of SPH1 and SPH2. b) 3D model of the clip domain-1 in PAP3 is highly similar to the known structure of PAP2 clip domain-1 (Huang et al., 2007). α helix, red; β strand, yellow; coil, green; Cys, pink. c) Phylogenetic analysis of the entire clip-domain SP/SPH sequences in groups A (black, SPH, group-3 clip domain), B (red, SP, group-2 clip domain), C (green, SP, group-1a clip domain), and D (blue, SP, group-1b or-1c clip domain). d) PAP3 and SP50 mRNA levels in M. sexta tissues from various life stages. X-axis, RNA-seq library number; Y-axes, FPKM values of SP50 (black dotted line) and PAP3 (red solid line). PAP3 transcripts are abundant in fat body of wandering larvae and early pupae; SP50 mRNA levels are high in fat body and ovary of late pupae and adults. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)