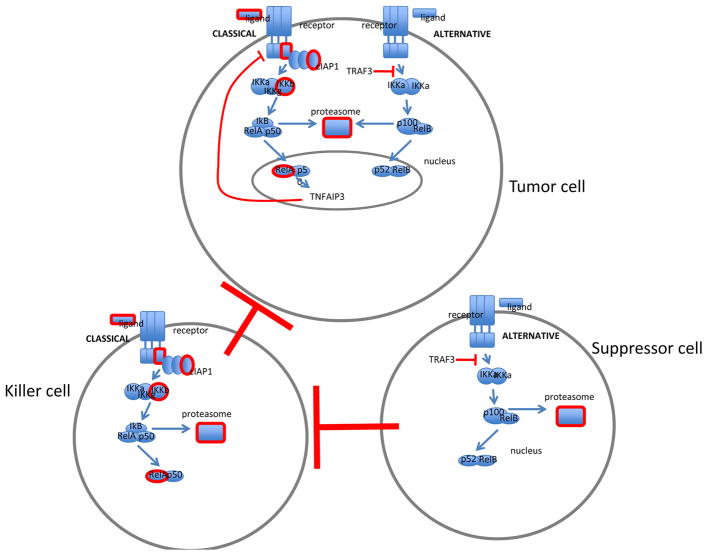
Figure 1.
Schematic of NF-kB signaling pathways. NF-kB transcription factors are activated in classical or alternative signaling pathways. Molecules that have preclinical and clinical inhibitors are highlighted in red. Much of the development for therapeutic targets has been focused on the classical signaling cascade. In the classical pathway, stimulation by TNF, for example, triggers IκB kinase (IKK)β to phosphorylate the inhibitory IκB proteins, leading to their rapid ubiquitination and proteasome-mediated degradation which culminates in the release of NF-κB complexes from their inhibitory interaction (5, 9, 45). As a result, NF-κB heterodimers p50/p65 translocate to the nucleus where they activate transcription of a plethora of genes involved in immune response, cell growth, and cell survival (2, 92, 93). The alternative NF-κB cascade is typically stimulated by Lymphotoxin β which triggers IKKα homodimers to phosphorylate p100/NF-κB2, resulting in proteasomal removal of an inhibitory C-terminal domain, and generating the NF-κB p52 subunit (94). Consequently, p52/RelB heterodimers preferentially accumulate in the nucleus, and activate transcription, based on subtleties in DNA motif preference (26).