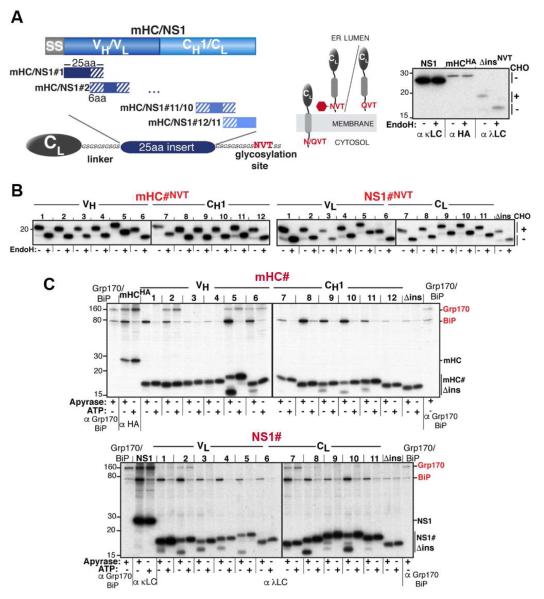
Figure 1. An in vivo peptide library reveals distinct substrate binding patterns for BiP and Grp170.
(A) A series of 25 amino acid overlapping peptides corresponding to the mHC and NS1 were inserted into a flexible GS-linker downstream of an ER-targeted λ LC constant domain (CL) ending with a C-terminal NVT glycosylation site or QVT. Only upon ER entry is the NVT site modified (red hexagon). EndoH resistance confirmed that NS1 and mHC are glycoproteins (right). (B) After transfection into COS-1 cells, metabolically labeled peptide constructs were immunoprecipitated with anti-λ antiserum, treated with (+) or without (−) EndoH and analyzed by SDS-PAGE. (C) COS-1 cells co-expressing Grp170, BiP, and the indicated peptide constructs were metabolically labeled, lysed with ATP or apyrase added. Peptide constructs were isolated with anti-λ and separated on SDS gels. Cells transfected with only Grp170 and BiP serve as markers and the full-length client for each set of peptides is included as a reference for chaperone binding.