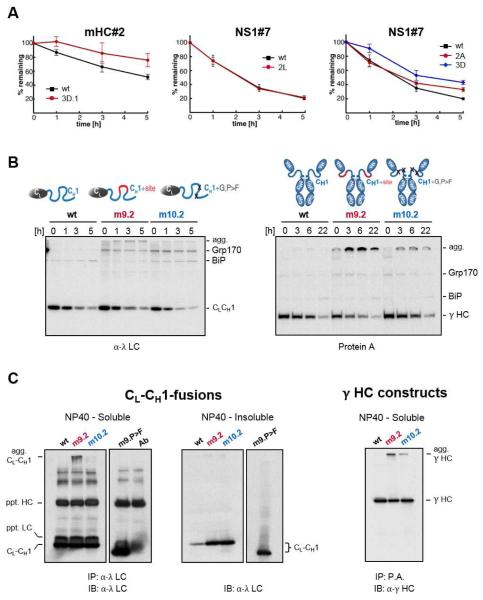
Figure 6. Introduction or disruption of (co-)chaperone binding sites alters the fate of client proteins in vivo.
(A) COS-1 cells expressing the indicated mHC#2 and NS1#7 constructs were pulse-labeled and chased for the indicated times. Immunoprecipitated peptide constructs were analyzed by SDS-PAGE, signals were quantified by phosphorimaging and the amount of remaining constructs over time was calculated ±SEM (n≥3). (B) Grp170 and BiP were co-expressed with the wild-type CL-CH1-fusion protein (left) and with the corresponding mutants in the full-length γ HC (right). Cells were pulse-labeled and chased for the indicated times. Immunoprecipitated material was analyzed by reducing SDS-PAGE and migration of clients and chaperones are marked. Slower migrating aggregated clients are indicated (agg). (C) COS-1 cells were co-transfected with Grp170, wild-type (D), and the indicated CL-CH1-fusion proteins. Cells were lysed in NP40 lysis buffer and soluble material from the indicated transfectants was immunoprecipitated with anti-λ (CL-CH1-fusion) and blotted with anti-λ antisera. The antibody control reveals bands present in the antiserum used for immunoprecipation, which are recognized when the same anti-λ serum is used for blotting. Equal amounts of NP40-insoluble material was directly analyzed by western blotting and the images of soluble and insoluble for each mutant are from the same exposure.