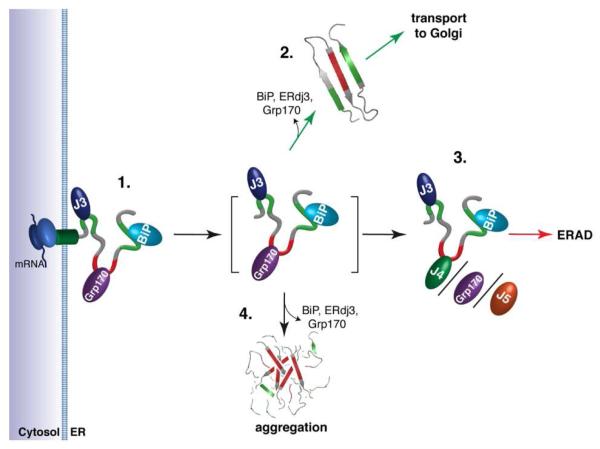
Figure 7. A model predicting how members of the ER Hsp70 chaperone system might interact to control the fate of client proteins.
(1.) Proteins entering the ER can encounter the (co-)chaperones ERdj3, BiP, and Grp170, which reside in proximity to the translocon. Grp170 recognizes sequences (indicated in red) that are distinct from BiP and ERdj3 binding sequences (indicated in green). (2.) As folding procedes, these (co-)chaperones are released allowing the properly matured client to be transported to the Golgi. Grp170/ERdj4/ERdj5 binding sites (indicated in red) become buried during folding, protecting them from aggregation. (3.) If folding fails, the sites recognized by Grp170/ERdj4/ERdj5 remain exposed, leading the client to be targeted for ERAD. (4.) Release of BiP, ERdj3, and Grp170 in the absence of folding can lead to aggregation. Thus, either rapid burial of the Grp170/ERdj4/ERdj5 recognition sites upon folding or their detection for the purpose of ERAD is critical, as these sites possess high aggregation propensity.