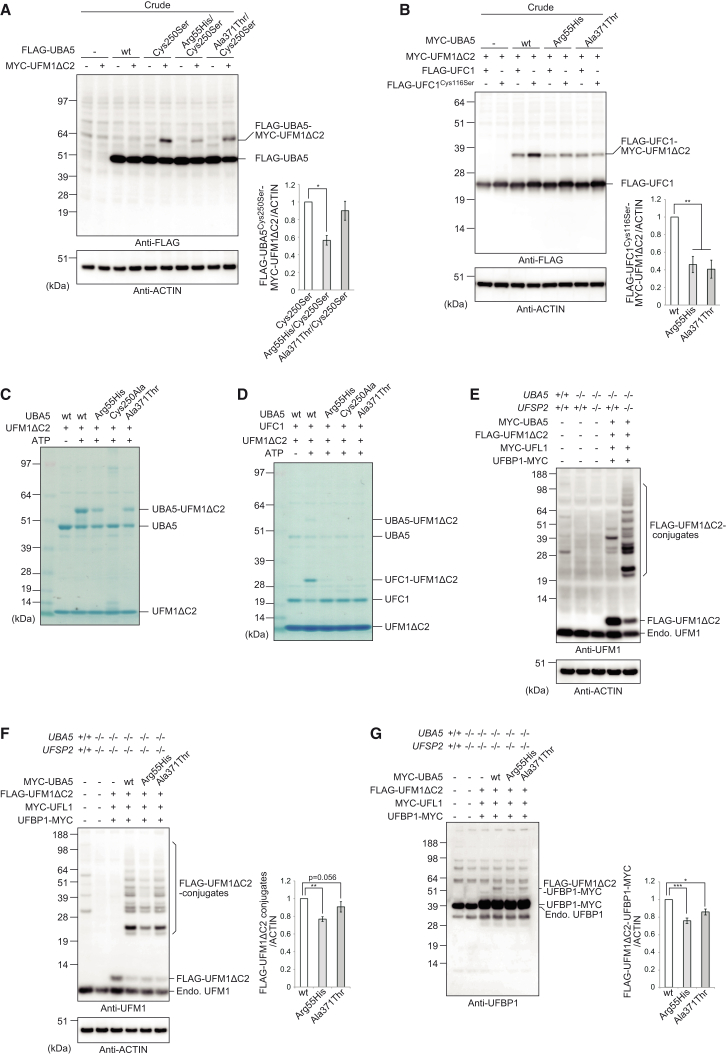
Figure 3.
Impaired Function of UBA5 Mutants
(A and B) Immunoblot assay of UBA5 mutant p.Cys250Ser and double mutants p.Arg55His/p.Cys250Ser and p.Ala371Thr/p.Cys250Ser in UBA5−/− HEK293T cells. Indicated constructs (0.1 μg for UBA5, 0.5 μg for UFC1, and 2 μg for UFM1ΔC2) were expressed in UBA5-deficient HEK293T cells. 24 hr after transfection, the cell lysates were subjected to immunoblot analysis with indicated antibodies as described in Figure 2B. Bar graphs indicate the quantitative densitometric analyses of UBA5-UFM1 and UFC1-UFM1 intermediates relative to ACTIN. Statistical analysis was performed using the unpaired t test (Welch test). The data represent the means ± SE of four separate experiments. ∗p < 0.05 and ∗∗p < 0.01.
(C and D) In vitro thioester formation assay of UFM1 by UBA5 (C) and of UFM1 by UFC1 (D). Recombinant GST-UFM1ΔC2, GST-UFC1, and GST-UBA5, as well as UBA5 mutants p.Arg55His (GST-UBA5Arg55His), p.Ala371Thr (GST-UBA5Ala371Thr), and p.Cys250Ala (negative control; GST-UBA5Cys250Ala) were produced in E. coli and the recombinant proteins were purified by chromatography on Glutathione Sepharose 4B (GE Healthcare UK). After digestion of GST by PreScission Protease (GE Healthcare UK), the recombinant proteins were dialyzed against 50 mM BisTris (pH 6.5), 100 mM NaCl, 10 mM MgCl2, and 0.1 mM DTT (reaction buffer). Most thioester formation reactions contained reaction buffer with 0.8 μg UFM1ΔC2 and some of the following: 5 mM ATP, 0.08 (for UFC1-UFM1 thioester formation assay) or 0.8 (for UBA5-UFM1 thioester formation assay) μg UBA5 or UBA5 mutants, and 0.8 μg UFC1. Reactions were incubated for 5 min at 25°C and stopped by the addition of NuPAGE-loading buffer lacking reducing agent, followed by 10 min incubation at 37°C, NuPAGE (4%–12% acrylamide gradient), and Coomassie brilliant blue staining. Data shown are representative of three separate experiments.
(E) Immunoblot assay to detect UFM1 conjugates. MYC-UBA5 (0.1 μg) was expressed in combination with indicated constructs (each 1 μg) in UBA5-deficient or UBA5-UFSP2 double-deficient HEK293T cells. Cells were lysed by 200 μL of TNE, and the lysate was then centrifuged at 10,000 × g for 10 min at 4°C to remove debris. The supernatant was subjected to immunoblot analyses with indicated antibodies.
(F) Immunoblot assay to study the effect of UBA5 mutants on UFM1 conjugate formation. MYC-UBA5 or MYC-UBA5 mutants (0.1 μg) were expressed in combination with indicated constructs (each 1 μg) in UBA5-UFSP2 double-deficient HEK293T cells. Cells were lysed by 200 μL of TNE, and the lysate was then centrifuged at 10,000 × g for 10 min at 4°C to remove debris. The supernatant was subjected to immunoblot analyses with indicated antibodies. Bar graph indicates the quantitative densitometric analyses of FLAG-UFM1 conjugates relative to ACTIN. Statistical analysis was performed using the unpaired t test (Welch test). The data represent the means ± SE of six separate experiments. ∗p < 0.05.
(G) Immunoblot assay to study the effect of UBA5 mutants on UFM1-UFBP1 conjugate formation. Transfection and subsequent immunoblot analysis were conducted as shown in (F). Bar graph indicates the quantitative densitometric analyses of FLAG-UFM1-UFBP1-MYC relative to ACTIN. Rabbit polyclonal anti-UFBP1 antibody6 was used for immunodetection. Statistical analysis was performed using the unpaired t test (Welch test). The data represent the means ± SE of six separate experiments. ∗p < 0.05 and ∗∗∗p < 0.001.