Figure 4.
Loss of UFM1 in Central Nervous System Causes Microcephaly
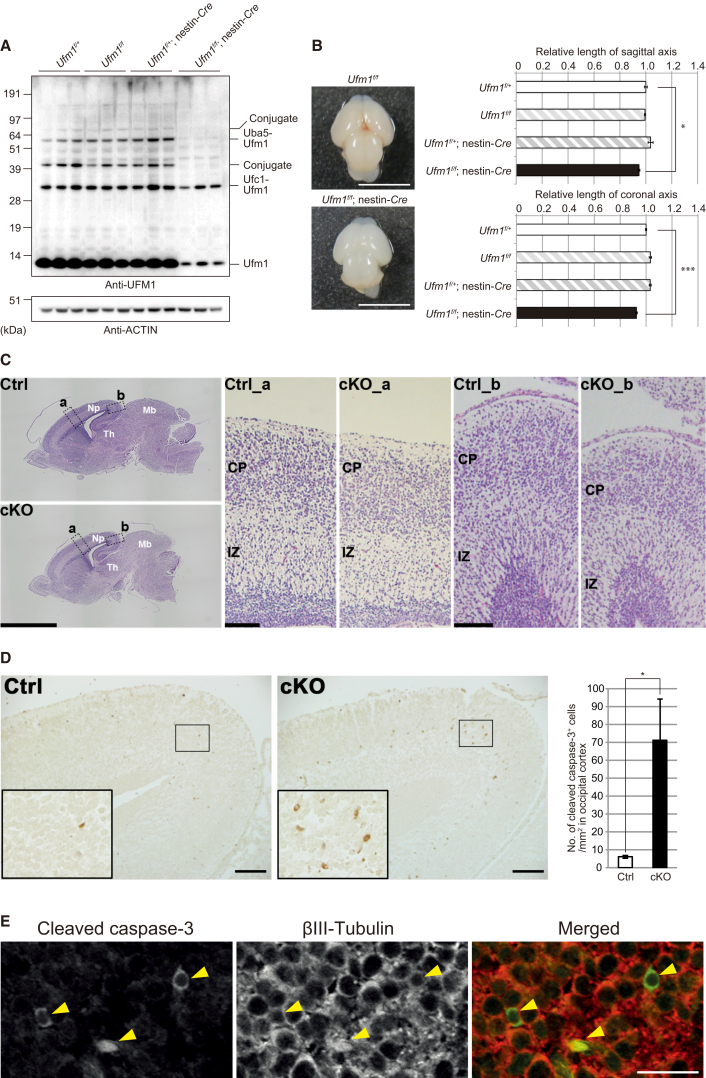
(A) Immunoblot analysis of UFM1 in mice with indicated genotypes. Mice were delivered by caesarean section at E18.5, and then mouse brains were homogenized in 0.25 M sucrose, 10 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) (pH 7.4), and 1 mM dithiothreitol (DTT). The homogenates were subjected to immunoblot analysis with indicated antibodies. Samples prepared from three mice with indicated genotype were loaded.
(B) A dorsal view of brains of Ufm1f/f and Ufm1f/f;nestin-Cre mice delivered by Caesarean section at E18.5. Graphs show axial distance (from the anterior edge of cerebrum to posterior edge of mid brain) and maximal lateral distance of brains of indicated genotype mice. Data presented as mean ± SE of Ufm1f/+ (n = 4), Ufm1f/f (n = 4), Ufm1f/+;nestin-Cre (n = 5), and Ufm1f/f;nestin-Cre (n = 6). Statistical analysis was performed using the unpaired t test. ∗p < 0.05 and ∗∗∗p < 0.001.
(C) Histological analyses of brains of Ufm1f/+;nestin-Cre (Ctrl) and Ufm1f/f;nestin-Cre (cKO) mice. Embryos at E18.5 were delivered by Caesarean section, and their heads were fixed by immersion in 0.1 M phosphate buffer (pH 7.4) containing 4% paraformaldehyde and 4% sucrose. Each brain was carefully dissected and processed for paraffin embedding, and then 3 μm sagittal sections were prepared for haematoxylin and eosin staining. Images were captured with BZ-9000 (Keyence) and BX51 microscopes (Olympus). Boxed regions a and b in the neopallium are magnified and shown on the right as indicated. Note that the occipital region (b) of neopallium in the mutant brain is thinner than that in control, while the difference in the parietal region (a) is less apparent. Scale bars are 2 mm and 0.1 mm. Abbreviations are as follows: Np, neopallium; Mb, midbrain; Th, thalamus; IZ, intermediate zone; CP, cortical plate.
(D) Apoptotic cells in the occipital region of neopallium of Ufm1f/+;nestin-Cre (Ctrl) and Ufm1f/f; nestin-Cre (cKO) mice at E18.5. Sections prepared as described in (C) were immunostained by rabbit polyclonal anti-cleaved caspase-3 antibody (Cell Signaling Technology [CST] cat# 9661, RRID: AB_2314091; 1:500) as described previously.28 Images were captured with BX53 microscope (Olympus). Each inset is a magnified image. Scale bars represent 100 μm. For quantification, the number of cleaved caspase-3-positive cells per unit area was calculated in each occipital cortex, which was defined as the cerebral cortex located posterior to the hippocampus. Statistical analysis was performed using the unpaired t test (n = 3 animals for each group). Data represent the means ± SE. ∗p < 0.05. The area was measured by NIH Image/ImageJ.
(E) Double-immunofluorescence analysis. Section of cKO brain (occipital region of neopallium) prepared as described in (C) was double-immunostained with anti-cleaved-caspase-3, mouse monoclonal anti-βIII Tubulin antibody (clone 5G8, Promega, 1:1,000), goat anti-mouse Alexa Fluor 594, and goat anti-rabbit Alexa Fluor 488 (Molecular Probes, 1:1,000). Images were captured with confocal FV1200 microscope (Olympus). Scale bar represents 20 μm.