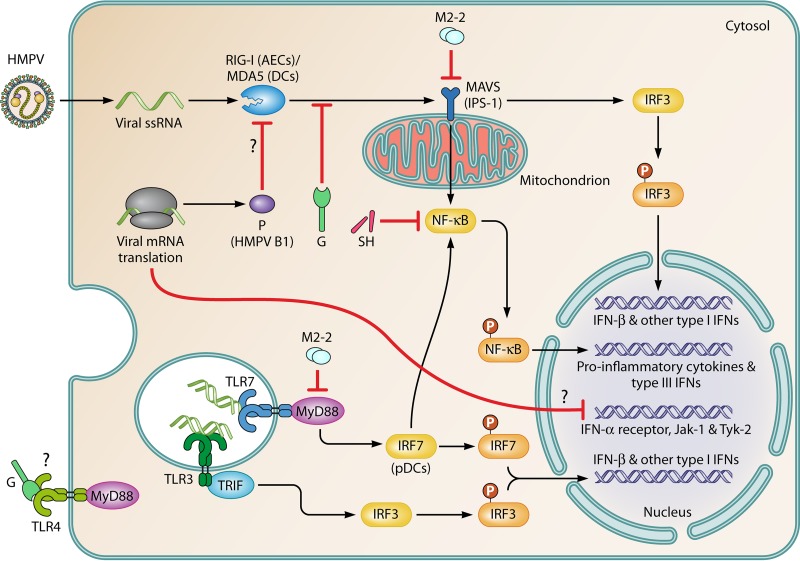
FIG 4.
Sensing of HMPV by different PRRs and modulation of the IFN response by viral proteins. AECs and DCs that inhabit the lower respiratory tract use two major pathways to sense HMPV replication. (Top) The first pathway involves RLRs, including RIG-I (more relevant in AECs) and MDA5 (more relevant in DCs) (155, 157). These RLRs couple MAVS for signaling, which leads to the secretion of type I IFNs. Proinflammatory cytokines and type III IFNs are also secreted through the activation of IRF3 and NF-κB, respectively. Products of viral replication, including cytosolic dsRNAs and uncapped 5′-triphosphates present in the genomic RNA, activate RLRs. (Bottom) The second pathway involves TLRs. TLR3, TLR4, and TLR7 interact with viral products. The activation of TLR3 (in most cells) and TLR7 (more important in pDCs) by viral dsRNAs leads to the secretion of type I IFNs by TIR domain-containing adaptor-inducing IFN-β (TRIF)/IRF3-dependent and MyD88/IRF7-dependent pathways, respectively, and the G protein impairs TLR4 signaling. HMPV proteins have synergizing/additive effects on the inhibition of viral sensing. The G and P (HMPV B1) proteins impair sensing of viral RNA species through the inhibition of RIG-I- and MAVS-dependent signaling (154, 155). The M2-2 and SH proteins are key virulence factors that negatively modulate signaling through both PRRs due to their interactions with the TLR adaptor MyD88 (167) and NF-κB (124), respectively. Furthermore, M2-2 directly interacts with MAVS, impairing the upregulation of IFN-β (156). Additionally, the G protein of HMPV has been proposed to interact with and abrogate signaling through TLR4 (158, 168), although the mechanism remains unknown. Moreover, HMPV infection leads to the downregulation of IFN-α receptors, Jak-1, and Tyk-2, thus impairing the autocrine IFN response in infected cells (165).