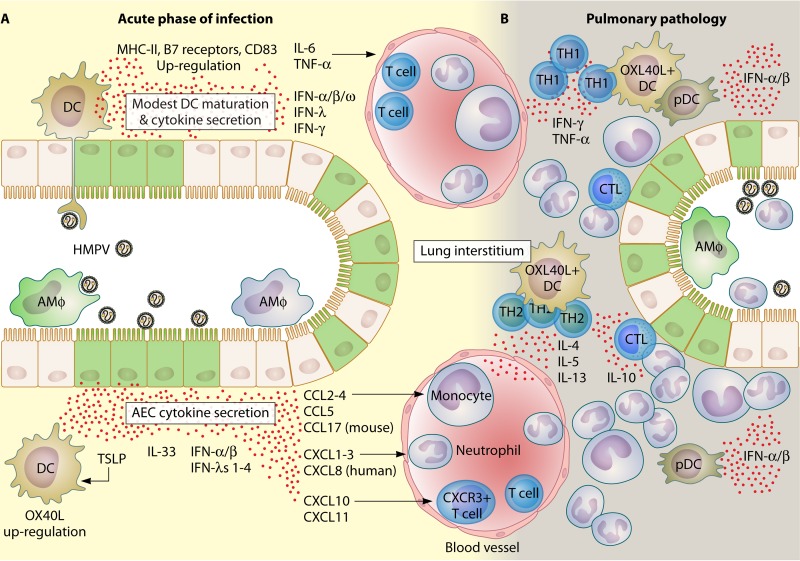
FIG 5.
Chain of events leading to pulmonary immunopathology following HMPV infection. Sensing of HMPV is the initial step that leads to pulmonary damage and inflammatory hyperresponsiveness. (A) During the acute phase of infection, AECs and DCs are the major populations that sense HMPV infections in the respiratory tract (149). Both infected AECs (green) and DCs secrete a vast repertoire of cytokines (148, 169, 186). Although DCs sense viral replication through PRRs, they develop only a poor to modest maturation response characterized by a mild upregulation of MHC-II, B7 receptors, and CD38. Chemokines bearing CC and CXC motifs are the most important cytokines that recruit and activate several immune cell subsets, including neutrophils, T cells, and monocytes, soon after initial exposure to HMPV. Alveolar macrophages (infected ones are shown in green) are important immune cells that promote pathology during the acute phase of infection. More specific effects have been attributed to the secretion of TSLP by HMPV-infected AECs. TSLP activates DCs (that upregulate OX40L) and leads to the activation and differentiation of T helper cells into a more pathogenic and poor antiviral phenotype (see panel B) (138, 171). (B) During established pulmonary pathology, TH1 cells (during the first 7 dpi) and TH2 cells (after 10 dpi) form perivascular infiltrates, where most cytokines that either impair viral clearance (IL-10) or promote and prolong airway inflammation (e.g., TNF-α, IL-6, IL-5, and IL-13) are secreted (220, 222). pDCs contribute to viral clearance by secreting type I IFNs. Infiltrating CTLs are the major effectors of viral clearance in infected tissue. Neutrophils are a dominant population that mediate the inflammation of HMPV-infected lungs and are also suspected targets for viral replication (138).