Abstract
AIM: To investigate the expression of matrix metalloproteinases (MMP), a group of proteolytic enzymes with a central role in extracellular matrix invasion and degradation, in stromal sarcomas. METHODS: 11 endometrial stromal sarcomas (four low grade tumours, seven high grade) were stained for MMP-2, MMP-3, and MMP-9 using immunohistochemical stains. The surgical material consisted of nine hysterectomy specimens and two pelvic recurrences. Three hysterectomy specimens, removed for leiomyomas, were studied as controls. Staining area was evaluated using image analysis. RESULTS: Age at the time of diagnosis ranged from 21 to 67 years. Four of the 11 patients (three with high grade tumours and one with a low grade tumour) died of the disease, six remained free of disease, and one was lost to follow up. Staining for MMP-2, MMP-3, and MMP-9 was more diffuse in high grade tumours than in low grade tumours and controls. Staining for MMP-3 and MMP-9 was more pronounced in high grade than in low grade tumours (p = 0.04; p = 0.05). Staining for MMP-9 was significantly greater in all stromal sarcomas than in controls (p < 0.001 for high grade tumours v controls; p < 0.01 for low grade tumours v controls). Diffuse staining for MMP-2, exceeding 90% of the tumour area, was observed in three of seven high grade tumours but in no low grade tumours. There was no apparent correlation between staining for any of the three enzymes and survival. CONCLUSIONS: Both low and high grade endometrial stromal tumours express matrix metalloproteinases. MMP-3 and MMP-9 are expressed more diffusely in high grade than in low grade tumours. In the individual case, diffuse staining for MMP-2 appears to best characterise the high grade tumours. Thus staining for MMP-2 may aid in differentiating high grade from low grade tumours, and MMP-9 in differentiating normal endometrial stroma from low and high grade endometrial stromal sarcomas. MMP expression does not appear to predict disease outcome in endometrial stromal sarcoma.
Full text
PDF
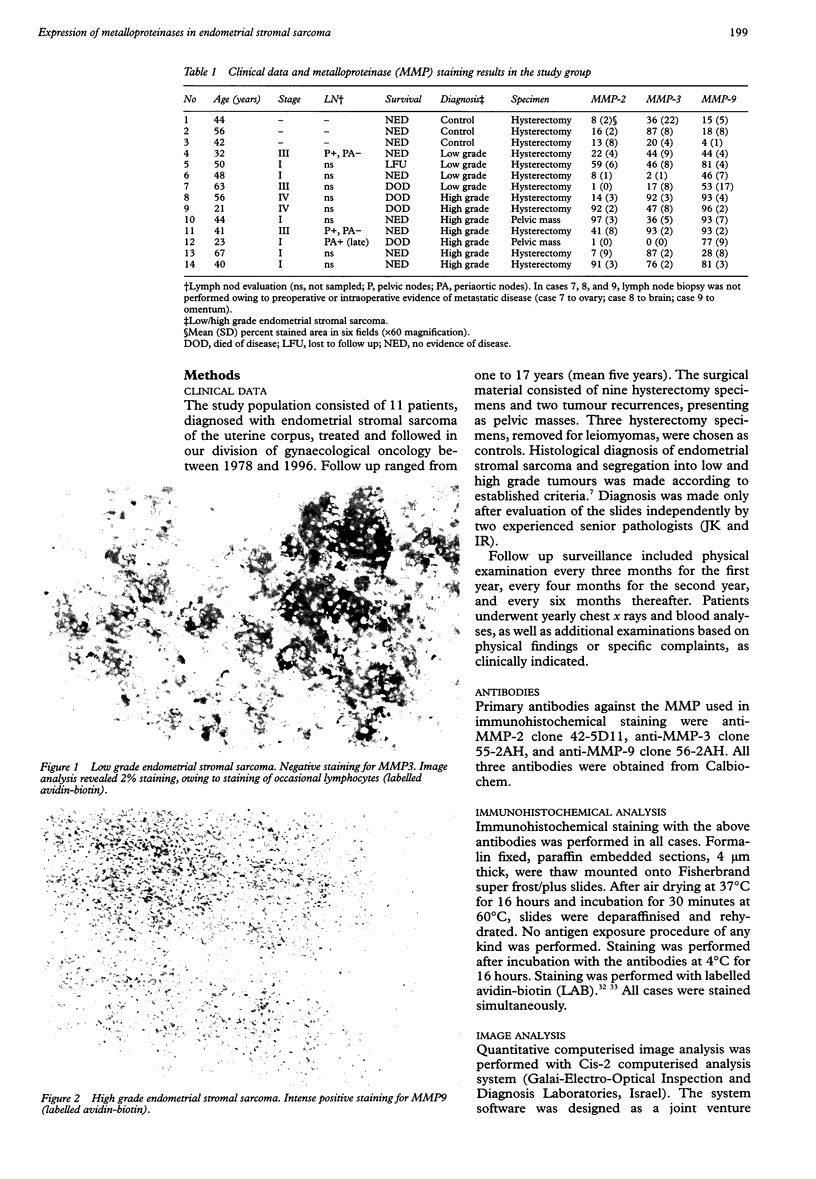



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aznavoorian S., Murphy A. N., Stetler-Stevenson W. G., Liotta L. A. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993 Feb 15;71(4):1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Benelli R., Adatia R., Ensoli B., Stetler-Stevenson W. G., Santi L., Albini A. Inhibition of AIDS-Kaposi's sarcoma cell induced endothelial cell invasion by TIMP-2 and a synthetic peptide from the metalloproteinase propeptide: implications for an anti-angiogenic therapy. Oncol Res. 1994;6(6):251–257. [PubMed] [Google Scholar]
- Brown P. D., Bloxidge R. E., Stuart N. S., Gatter K. C., Carmichael J. Association between expression of activated 72-kilodalton gelatinase and tumor spread in non-small-cell lung carcinoma. J Natl Cancer Inst. 1993 Apr 7;85(7):574–578. doi: 10.1093/jnci/85.7.574. [DOI] [PubMed] [Google Scholar]
- Chang K. L., Crabtree G. S., Lim-Tan S. K., Kempson R. L., Hendrickson M. R. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990 May;14(5):415–438. doi: 10.1097/00000478-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Cheung A. N., Tin V. P., Ngan H. Y., Chung L. P., Khoo U. S. Interphase cytogenetic study of endometrial stromal sarcoma by chromosome in situ hybridization. Mod Pathol. 1996 Sep;9(9):910–918. [PubMed] [Google Scholar]
- Crawford H. C., Matrisian L. M. Tumor and stromal expression of matrix metalloproteinases and their role in tumor progression. Invasion Metastasis. 1994;14(1-6):234–245. [PubMed] [Google Scholar]
- Dabbs D. J., Silverman J. F., Geisinger K. R. Immunohistochemical study of uterine stromal sarcoma and rhabdomyosarcoma. Arch Pathol Lab Med. 1989 Oct;113(10):1151–1154. [PubMed] [Google Scholar]
- De Fusco P. A., Gaffey T. A., Malkasian G. D., Jr, Long H. J., Cha S. S. Endometrial stromal sarcoma: review of Mayo Clinic experience, 1945-1980. Gynecol Oncol. 1989 Oct;35(1):8–14. doi: 10.1016/0090-8258(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Dunton C. J., Kelsten M. L., Brooks S. E., Viglione M. J., Carlson J. A., Mikuta J. J. Low-grade stromal sarcoma: DNA flow cytometric analysis and estrogen progesterone receptor data. Gynecol Oncol. 1990 May;37(2):268–275. doi: 10.1016/0090-8258(90)90346-m. [DOI] [PubMed] [Google Scholar]
- Evans H. L. Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer. 1982 Nov 15;50(10):2170–2182. doi: 10.1002/1097-0142(19821115)50:10<2170::aid-cncr2820501033>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Skubitz A. P., Fields G. B. Tumor cell invasion, matrix metalloproteinases, and the dogma. Lab Invest. 1994 Jun;70(6):781–783. [PubMed] [Google Scholar]
- Gilles C., Polette M., Seiki M., Birembaut P., Thompson E. W. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997 May;76(5):651–660. [PubMed] [Google Scholar]
- Giorno R. A comparison of two immunoperoxidase staining methods based on the avidin-biotin interaction. Diagn Immunol. 1984;2(3):161–166. [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Himelstein B. P., Canete-Soler R., Bernhard E. J., Dilks D. W., Muschel R. J. Metalloproteinases in tumor progression: the contribution of MMP-9. Invasion Metastasis. 1994;14(1-6):246–258. [PubMed] [Google Scholar]
- Hua J., Muschel R. J. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996 Nov 15;56(22):5279–5284. [PubMed] [Google Scholar]
- Kobayashi M., Hamada J., Li Y. Q., Shinobu N., Imamura M., Okada F., Takeichi N., Hosokawa M. A possible role of 92 kDa type IV collagenase in the extramedullary tumor formation in leukemia. Jpn J Cancer Res. 1995 Mar;86(3):298–303. doi: 10.1111/j.1349-7006.1995.tb03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz-Mercer B., Czernobilsky B., Dgani R., Dallenbach-Hellweg G., Moll R., Franke W. W. Immunocytochemical study of an endometrial diffuse clear cell stromal sarcoma and other endometrial stromal sarcomas. Cancer. 1987 Apr 15;59(8):1494–1499. doi: 10.1002/1097-0142(19870415)59:8<1494::aid-cncr2820590817>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lillemoe T. J., Perrone T., Norris H. J., Dehner L. P. Myogenous phenotype of epithelial-like areas in endometrial stromal sarcomas. Arch Pathol Lab Med. 1991 Mar;115(3):215–219. [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Moses M. A., Shing Y. Production of matrix metalloproteinases and a metalloproteinase inhibitor by swarm rat chondrosarcoma. Biochem Biophys Res Commun. 1994 Feb 28;199(1):418–424. doi: 10.1006/bbrc.1994.1245. [DOI] [PubMed] [Google Scholar]
- Nola M., Babić D., Ilić J., Marusić M., Uzarević B., Petrovecki M., Sabioncello A., Kovac D., Jukić S. Prognostic parameters for survival of patients with malignant mesenchymal tumors of the uterus. Cancer. 1996 Dec 15;78(12):2543–2550. doi: 10.1002/(sici)1097-0142(19961215)78:12<2543::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Nordal R. R., Kristensen G. B., Kaern J., Stenwig A. E., Pettersen E. O., Tropé C. G. The prognostic significance of surgery, tumor size, malignancy grade, menopausal status, and DNA ploidy in endometrial stromal sarcoma. Gynecol Oncol. 1996 Aug;62(2):254–259. doi: 10.1006/gyno.1996.0224. [DOI] [PubMed] [Google Scholar]
- Norris H. J., Taylor H. B. Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer. 1966 Jun;19(6):755–766. doi: 10.1002/1097-0142(196606)19:6<755::aid-cncr2820190604>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P. B., Simsir A., Valea F., French D. L. Correlation of the in situ detection of polymerase chain reaction-amplified metalloproteinase complementary DNAs and their inhibitors with prognosis in cervical carcinoma. Cancer Res. 1995 Jan 15;55(2):267–275. [PubMed] [Google Scholar]
- Okada Y., Tsuchiya H., Shimizu H., Tomita K., Nakanishi I., Sato H., Seiki M., Yamashita K., Hayakawa T. Induction and stimulation of 92-kDa gelatinase/type IV collagenase production in osteosarcoma and fibrosarcoma cell lines by tumor necrosis factor alpha. Biochem Biophys Res Commun. 1990 Sep 14;171(2):610–617. doi: 10.1016/0006-291x(90)91190-4. [DOI] [PubMed] [Google Scholar]
- Rifas L., Fausto A., Scott M. J., Avioli L. V., Welgus H. G. Expression of metalloproteinases and tissue inhibitors of metalloproteinases in human osteoblast-like cells: differentiation is associated with repression of metalloproteinase biosynthesis. Endocrinology. 1994 Jan;134(1):213–221. doi: 10.1210/endo.134.1.8275936. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Nakayama Y., Ohtani K., Seiki M., Satoh K. Pulmonary metastatic lesion of endolymphatic stromal myosis expresses metastasis-related genes but not invasion-related matrix type metalloproteinase. Cancer Lett. 1997 Jan 30;112(2):245–249. doi: 10.1016/s0304-3835(96)04580-6. [DOI] [PubMed] [Google Scholar]
- Sato H., Kida Y., Mai M., Endo Y., Sasaki T., Tanaka J., Seiki M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992 Jan;7(1):77–83. [PubMed] [Google Scholar]
- Shima I., Sasaguri Y., Kusukawa J., Yamana H., Fujita H., Kakegawa T., Morimatsu M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer. 1992 Dec 15;70(12):2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stack M. S., Ellerbroek S. M., Fishman D. A. The role of proteolytic enzymes in the pathology of epithelial ovarian carcinoma. Int J Oncol. 1998 Mar;12(3):569–576. doi: 10.3892/ijo.12.3.569. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996 May;148(5):1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Tosi P., Sforza V., Santopietro R. Estrogen receptor content, immunohistochemically determined by monoclonal antibodies, in endometrial stromal sarcoma. Obstet Gynecol. 1989 Jan;73(1):75–78. [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24(3):209–218. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- Wolfson A. H., Wolfson D. J., Sittler S. Y., Breton L., Markoe A. M., Schwade J. G., Houdek P. V., Averette H. E., Sevin B. U., Penalver M. A multivariate analysis of clinicopathologic factors for predicting outcome in uterine sarcomas. Gynecol Oncol. 1994 Jan;52(1):56–62. doi: 10.1006/gyno.1994.1011. [DOI] [PubMed] [Google Scholar]






