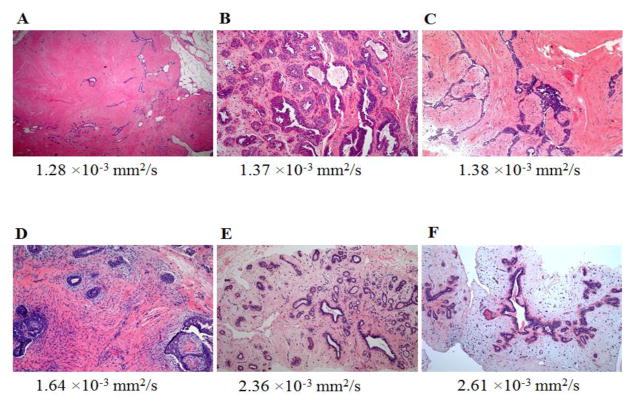
Fig. 2.
Hematoxylin-eosin stained slides of representative fibroadenomas with original magnification ×100 and their corresponding ADC values:
A, 65-year-old woman with personal history of right-breast invasive ductal carcinoma, status post lumpectomy with an MRI-detected fibroadenoma in the left breast exhibiting no epithelial hyperplasia, low stromal cellularity, no immune cell infiltration and dense stroma.
B, 49-year-old woman with personal history of right-breast invasive ductal carcinoma, status post lumpectomy, with an MRI-detected fibroadenoma in the left breast exhibiting epithelial hyperplasia, high stromal cellularity, no immune cell infiltration and dense stroma.
C, 48-year-old woman with personal history of left-breast lobular carcinoma in situ, status post lumpectomy, with an MRI-detected fibroadenoma in the right breast exhibiting epithelial hyperplasia, low stromal cellularity, no immune cell infiltration and dense stroma.
D, 35-year-old woman with personal history of left-breast ductal carcinoma in situ, status post lumpectomy, with an MRI-detected fibroadenoma in the right breast exhibiting epithelial hyperplasia, high stromal cellularity, no immune cell infiltration and dense stroma.
E, 35-year-old woman undergoing neoadjuvant chemotherapy for right breast inflammatory ductal carcinoma, with an MRI-detected fibroadenoma in the left breast exhibiting no epithelial hyperplasia, low stromal cellularity, no immune cell infiltration and sparse stroma.
F, 40-year-old high-risk woman with an MRI-detected fibroadenoma in the left breast exhibiting epithelial hyperplasia, low stromal cellularity, immune cell infiltration and sparse stroma.