Abstract
Leukemia can promote T cell dysfunction and exhaustion that contributes to increased susceptibility to infection and mortality. The treatment-independent mechanisms that mediate leukemia-associated T cell impairments are poorly understood, but metabolism tightly regulates T cell function and may contribute. Here we show that B cell leukemia causes T cells to become activated and hypo-responsive with increased PD-1 and TIM3 expression similar to exhausted T cells and that T cells from leukemic hosts become metabolically impaired. Metabolic defects included reduced Akt/mTORC1 signaling, decreased expression of the glucose transporter Glut1 and Hexokinase 2 (HK2) and reduced glucose uptake. These metabolic changes correlated with increased Treg frequency and expression of PD-L1 and Gal-9 on both leukemic and stromal cells in the leukemic microenvironment. PD-1, however, was not sufficient to drive T cell impairment, as in vivo and in vitro anti-PD-1 blockade on its own only modestly improved T cell function. Importantly, impaired T cell metabolism directly contributed to dysfunction, as a rescue of T cell metabolism by genetically increasing Akt/mTORC1 signaling or expression of Glut1 partially restored T cell function. Enforced Akt/mTORC1 signaling also decreased expression of inhibitory receptors TIM3 and PD-1, and partially improved anti-leukemia immunity. Similar findings were obtained in T cells from patients with acute or chronic B cell leukemia, which were also metabolically exhausted and had defective Akt/mTORC1 signaling, reduced expression of Glut1 and HK2, and decreased glucose metabolism. Thus, B cell leukemia-induced inhibition of T cell Akt/mTORC1 signaling and glucose metabolism drives T cell dysfunction.
Keywords: Leukemia, T cell, Exhaustion, Metabolism, Glut1, Akt, mTOR
Introduction
Immunological defects are frequent complications and lead to increased susceptibility to infections in B cell leukemia patients (1, 2). Chemotherapeutic treatments, such as those provided in acute leukemia, can reduce production of hematopoietic cells and disrupt bone marrow niches to contribute to reduced immune function. Furthermore, targeted therapies for chronic B cell malignancies can have major impacts on non-leukemic immune cells (3). In addition, differentiated immune cells can also be actively suppressed prior to therapy (4–7). Despite the distinct natures of B cell derived acute lymphoblastic and chronic lymphocytic leukemia, each can lead to T cell dysregulation independent of treatment (7–9) that resembles of functional impairment and exhaustion of T cells chronically stimulated with viral antigen (10, 11). T cell exhaustion is thought to be important to prevent excessive inflammation and tissue damage and is marked by phenotypic changes including expression of inhibitory receptors such as PD-1 and TIM3 as well as decreased activation, proliferation, and inflammatory cytokine production upon restimulation (12, 13). In B cell malignancies, broad T cell stimulation (14, 15) and aberrant expression of immunomodulatory ligands or cytokines by leukemic and stromal cells may also promote T cell exhaustion (16, 17). The molecular mechanisms by which B cell leukemia promotes T cell dysfunction, however, are uncertain.
Metabolic pathways must be tightly regulated to allow normal proliferation and T cell effector function. Altered metabolic regulation may thus contribute to impaired T cell function in leukemia. While resting T cells utilize an oxidative metabolism, T cell activation promotes effector T cells (Teff) to induce the glucose transporter Glut1 and Hexokinase 2 (HK2) and upregulate a program termed aerobic glycolysis (18). The Phosphatidylinositide 3-kinase (PI3K)/Akt/mTORC1 pathway is critical in this metabolic reprogramming and activation of Akt/mTORC1 drives expression and cell surface trafficking of Glut1 (19, 20) as well as promote glycolytic metabolism (21). Consistent with a key role for aerobic glycolysis in Teff, Glut1-deficient T cells have impaired proliferation and are unable to efficiently elicit inflammation in vivo (22). As T cells differentiate into functionally distinct subsets, however, each population is metabolically unique. In particular, CD4+ regulatory T cells (Treg) primarily utilize oxidative metabolism and can be immune suppressive independent of PI3K/Akt/mTOR signaling and Glut1 (22, 23). Pathways that impair T cell metabolic reprogramming and induction of Glut1 will thus prevent effector T cell proliferation and function. Indeed, inhibition of T cell glycolysis can promote anergy and expression of PD-1 that are consistent with T cell exhaustion (24, 25). Conversely, PD-1 ligation has been shown to inhibit glycolysis and promote lipid oxidation (26, 27). It is however unknown, whether changes in T cell metabolism contribute to T cell dysfunction in leukemia.
Here we examine the mechanism of B cell leukemia-associated T cell dysfunction and show that inhibition of T cell metabolism contributes to impaired T cell function in both acute and chronic B cell leukemia. We show that functional exhaustion of T cells from leukemic hosts occurs with reduced ability of T cells to activate Akt/mTORC1 signaling and upregulate Glut1 and aerobic glycolysis. Importantly, restoring T cell metabolism through Akt activation or expression of Glut1 was sufficient to improve T cell function and activation of Akt in T cells delayed progression of leukemia. Together, these data demonstrate that inhibition of T cell glucose metabolism is a mechanism by which leukemia promotes T cell dysfunction. Restoring T cell metabolism may therefore provide a new avenue to promote immunological function in leukemia.
Materials and Methods
Mice
C57BL/6J and BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). T cell specific Glut1 transgenic (Glut1 tg) and myristoylated Akt (mAkt) mice on the C57BL/6J background were previously described and metabolically characterized (28, 29). Because FL5.12 cells were generated on the BALB/c background (30), mice were crossed with BALB/c and (C57BL/6J x BALB/c) F1 mice were used as hosts for FL5.12 cell transfers. Mice were bred and housed under specific pathogen-free conditions at Duke University Medical Center. All experiments were performed under protocols approved by the Institutional Animal Care and Use Committee. Six- to eight-week-old transgenic or non-transgenic littermates were used for all experiments.
FL5.12 Leukemia Model
Murine Pro-B-cell FL5.12 cells retrovirally transduced with MSCV-BCR/Abl-IRES-GFP were cultured in RPMI with 10% fetal calf serum (Gemini) as described (31) and tested mycoplasma negative. In some experiments 0.03ug/mL IFNγ (eBioscience) was added to culture media to induce inhibitory ligands. For in vivo experiments, cells were washed in PBS and 0.05–0.1 × 106 cells were injected intravenously. For immunization experiments, 0.02–1 × 106 BCR/Abl FL5.12 cells were irradiated (30 Gy) and injected subcutaneously seven days prior i.v. injections. At specified time points, splenocytes were isolated and red blood cells lysed using ACK buffer (Lonza). For anti-PD-1 treatment experiments, mice were immunized with irradiated FL5.12 cells seven days prior injection of live cells. After injection of leukemic cells, mice were treated with i.p. administration of PD-1 blocking antibody (250 μg/mouse) or isotype control every three days for the course of 12 days.
Patients and Blood Samples
Peripheral blood mononuclear cells from 37 CLL patients [32 patients in cohort 1 (Duke University, Durham, NC) and 5 patients in cohort 2 (Academic Medical Center, Amsterdam, The Netherlands)] and healthy donors, and bone marrow mononuclear cells from 5 B-ALL patients (Vanderbilt University, Nashville, TN) were isolated by density gradient centrifugation. All subjects were de-identified and gave written informed consent according to protocols approved by Institutional Review Boards of the collecting center in accordance with the Declaration of Helsinki. The diagnosis and staging (using the Rai system) of CLL were determined according to IWCLL criteria (32). Samples were analyzed fresh (CLL cohort 1) or after cryopreservation (CLL cohort 2 and ALL). Where fresh samples were analyzed for in vitro activation and metabolic assays, the distribution of patient subgroups in relation to disease characteristics was not normalized.
T Cell Purification and Stimulation
Human PBMCs and murine splenocytes were cultured in complete RPMI with 10% FBS, 1% Pen-Strep and stimulated by addition of anti-CD3 with or without anti-CD28 to cell cultures (eBioscience). For selected experiments, murine splenocytes were cultured with 0.03ug/mL IFNγ (eBioscience). In some cases, T cells were purified using pan T cell isolation kits (Miltenyi Biotec) and stimulated with plate-bound anti-CD3 and anti-CD28 (eBioscience) or anti-CD3, anti-CD2 and anti-CD28 coated beads (Miltenyi Biotec).
Flow Cytometry
Expression of T cell surface markers was measured by flow cytometry (MacsQuant, Miltenyi Biotec) and analyzed with FlowJo software (Tree Star, Ashland, OR). Following anti-human antibodies were used: CD4 Violet Blue (VB), CD25 Phycoerythrin (PE), CD8 Peridinin chlorophyll (PerCP; all Miltenyi Biotec), CD69 Fluorescein Isothiocyanate (FITC; BD), CD8 Allophycocyanin (APC), CD19 VB, CD71 APC, CTLA4 PE, CD200 FITC, CD200R PE, Gal9 PerCP, PD-L1 APC, PD-1 APC, TIM3 FITC, LAG3 PerCP, BTLA FITC, CD160 FITC, CD244 APC (all eBioscience). Following anti-mouse antibodies were used: Galectin-9 PerCP (Biolegend), CD4 VB, CD8 PerCP, CD8 APC, CD69 APC, CD25 PE, CD71 PE, CTLA4 PE, CD200 FITC, CD200R PE, Gal9 PerCP, PD-L1 APC, PD-1 PerCP, TIM3 APC, LAG3 PE, BTLA FITC, CD160 FITC, CD244 APC (all eBioscience). Glut1, HK2 and pS6 was measured by intracellular flow cytometry of PFA-fixed and methanol permeabilized cells using monoclonal rabbit anti-Glut1 (Abcam; ab652), anti-HK2 (Abcam, 131196) and anti-pS6 (Cell Signaling, 4858) in the presence of rat serum and Fc block, followed by anti-rabbit PE. Intracellular cytokine production was measured after 5-hour stimulation with PMA (50 ng/ml, Sigma-Aldrich) and ionomycin (750 ng/ml, Calbiochem) in the presence of GolgiPlug (eBioscience). Cells were permeabilized with Cytofix/Cytoperm Plus (BD) and stained with IL-2 PE and IFNγ APC (BD). Transcription factor staining was performed using the Mouse Regulatory T Cell Staining Kit (eBioscience) and FOXP3 PE antibodies (eBioscience). Cell proliferation was measured using CellTrace Violet (CTV; Invitrogen).
Metabolic Assays
Glycolysis measurements were normalized to cell number and have been described previously (19). Glucose uptake was measured using fluorescent glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) as described previously (33). Briefly, cells were cultured in glucose free media and incubated in 50μM 2-NBDG for 45 min. Fluorescence of selected T cell subpopulations was measured by flow cytometry.
Statistics
Data involving two groups were analyzed using paired or unpaired two-tailed Student’s t-test. Data of more than two groups were analyzed using two-way ANOVA with Bonferroni post-test. Statistical analysis was performed using Prism (Graphpad Software, La Jolla, CA, USA).
Results
Experimental B cell lymphoblastic leukemia induces T cell metabolic dysfunction in vivo
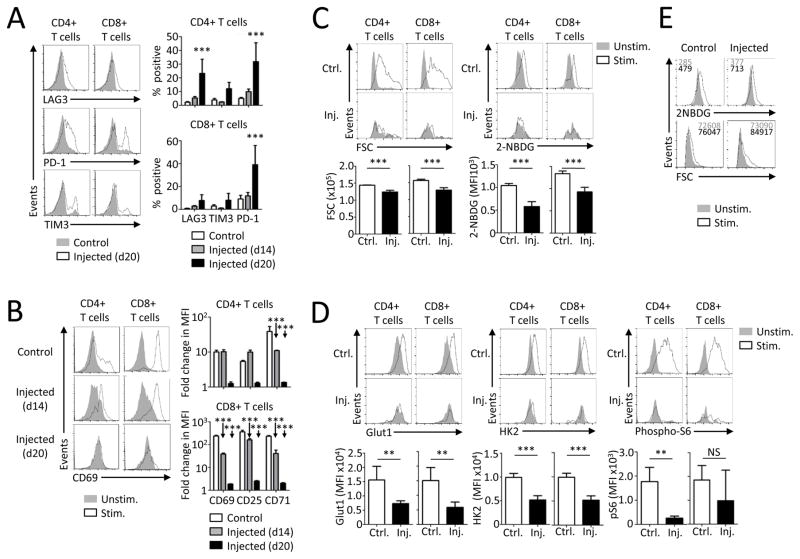
B cell leukemia can impair T cell function, but the molecular mechanisms and if changes in T cell metabolism contribute to T cell dysfunction were unknown. To address this gap, a murine model of B cell leukemia was employed in which BCR/Abl transduced murine pro-B cell FL5.12 cells were adoptively transferred into syngeneic immunocompetent hosts. Leukemic B cells accumulated in unimmunized mice and led to decreased survival (Supplemental Fig. 1A). If immunized with irradiated FL5.12 cells prior to transfer of live leukemic cells, however, recipients were capable to reject the leukemia, demonstrating potential of immune regulation. Indeed, non-irradiated leukemic B cells did alter endogenous T cells, as the percentage of activated, CD25, CD69 and CD71 positive splenic T cells increased as leukemia progressed (Supplemental Fig. 1B). Expression of inhibitory receptors was next measured on T cells from leukemia-bearing animals. Increased expression of PD-1 was measured on CD4+ and CD8+ T cells. In addition, CD4+ T cells showed elevated LAG3 expression and both CD4+ and CD8+ T cells showed a trend towards increased TIM3 expression (Fig. 1A).
FIGURE 1. Experimental B cell lymphoblastic leukemia induces T cell exhaustion and metabolic dysfunction in vivo.
Immunocompetent BALB/c mice were injected i.v. with BCR/Abl FL5.12 and splenic T cells were analyzed on indicated days. (A) Expression of specified markers was measured on T cells from not injected animals (Control, n=4) or T cells from animals 14 or 20 days after leukemia transfer (Injected, n=3–4/group) by flow cytometry. (B) T cells from control animals (n=2) or animals injected with leukemic cells (n=3/group) were stimulated with anti-CD3 over-night and the expression of specified markers was analyzed by flow cytometry. (C,D) 2-NBDG incorporation, Glut1, HK2 expression and S6 phosphorylation were analyzed by flow cytometry in resting or stimulated splenic T cells from control or injected mice on day 20 (n=4–5/group). The bar graphs represent values from stimulated samples. (E) CD8+ T cells were stimulated on anti-CD3 and anti-CD28 coated plates after enrichment by magnetic bead isolation and flow-sorting. Glucose uptake and cell size was measured by flow cytometry on day 1. Data are representative of two samples, each pooled from 3 leukemic or control mice. Data are representative of a minimum of one (A and B, day 14), two (B, day 20), or three (C,D) independent experiments. Data are mean ± s.d. (A,C,D) or s.e.m. (B). * P < 0.05, ** P < 0.01 and *** P < 0.001.
T cells from leukemia-bearing mice became functionally impaired independent of any treatment and failed to respond to in vitro activation over time. Consistent with in vivo T cell activation, CD69, CD25 and CD71 each showed progressively impaired in vitro re-induction as leukemia progressed (Fig. 1B). The proliferative capacity of T cells was also reduced, as both CD4+ and CD8+ T cells from leukemia-bearing mice became incapable of robust proliferation upon in vitro stimulation (Supplemental Fig. 1C). Importantly, splenic T cells isolated from mice injected with FL5.12 leukemia were metabolically affected and failed to increase cell size and glucose uptake upon in vitro anti-CD3 stimulation (Fig. 1C). These defects correlated with a failure of stimulated T cells from leukemia-bearing animals to induce Glut1 and HK2 or activate the Akt/mTORC1 pathway and phosphorylate S6 (Fig. 1D). Nevertheless, the leukemic environment was required for the persistence of the T cell metabolic dysfunction, as T cells purified and removed from leukemic cells showed elevated stimulation-induced glucose uptake and cell size comparable to controls (Fig. 1E). Collectively, these data show that leukemic cells alter the T cell microenvironment to induce T cell stimulation and expression of inhibitory receptors and activation markers that correlate with impaired T cell function and metabolism after restimulation.
Inhibitory ligands and regulatory T cells are present in leukemic microenvironment
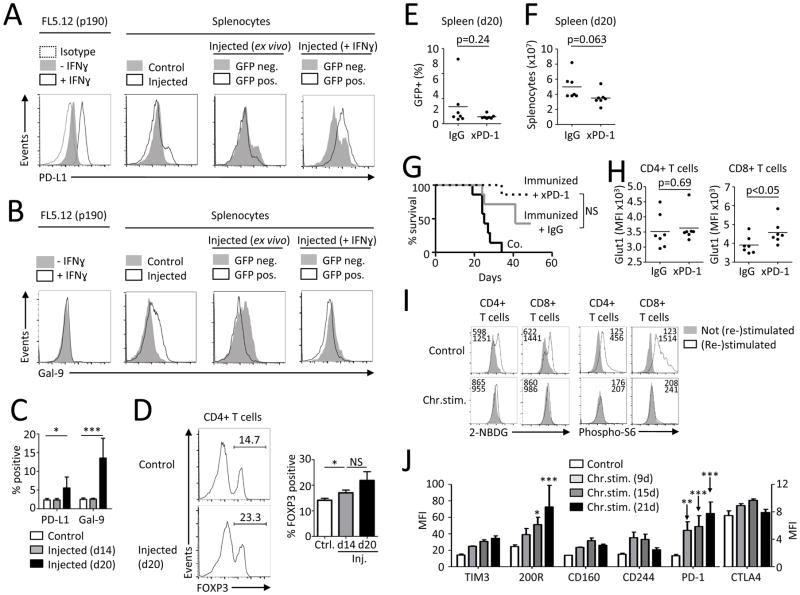
The failure of T cells from leukemia-bearing animals to properly activate upon secondary stimulation may have been due to insufficient activation or altered co-stimulation provided by leukemic and stromal cells. However, leukemic cells (GFP+) and stromal cells expressed similar levels of B7-1, B7-2, and B7-H2 as splenocytes not exposed to leukemia (Supplemental Fig. 1D). Further, increased TCR activation, CD28 co-stimulation, or addition of IL2 each failed to restore T cell function from leukemia-bearing mice when stimulated in the presence of leukemic cells (Supplemental Fig. 1E-H). Inhibitory pathways, such as PD-1/PD-L1, can modulate PI3K/Akt/mTOR signaling (27, 34) and may contribute to T cell metabolic dysfunction in leukemia. The expression of PD-L1 and Gal-9, a TIM3 ligand, was analyzed on leukemic cells and surrounding non-cancerous cells. IFNγ can induce PD-L1 (35) and BCR/Abl FL5.12 cells expressed low levels of PD-L1 that increased upon IFNγ stimulation (Fig. 2A). A population of PD-L1-high cells was found in spleens from leukemia-bearing animals that was comprised of both GFP+ leukemic and GFP− non-leukemic cells. Gal-9 was similarly expressed on splenocytes from leukemia-bearing mice and was predominantly from GFP− non-leukemic cells (Fig. 2B). The percentage of PD-L1 and Gal-9 positive splenic cells increased over time in animals with leukemia (Fig. 2C). Inhibitory ligands can also induce suppressive Treg cells (36, 37) and spleens of mice injected with FL5.12 leukemic cells were analyzed to determine if leukemia promoted Treg cells. Consistent with induction of inhibitory signals, the percentage of FOXP3+ cells among CD4+ T cells increased as leukemia progressed (Fig. 2D).
FIGURE 2. Leukemia associated T cell dysfunction is correlated with immunosuppressive micro-environment but can be recapitulated by chronic in vitro stimulation.
(A,B) Expression of PD-L1 and Gal-9 on FL5.12 cells in vitro and on splenocytes from control mice (n=2) or injected mice (n=5) ex vivo or after over-night culture with or without IFNγ. (C) Expression of PD-L1 and Gal-9 on control splenocytes (n=6) or splenocytes from mice 14 days (n=3) or 20 days (n=4) after injection of FL5.12 cells. (D) FOXP3 expression in CD4+ splenic T cells from control mice (n=2) or injected mice (n=3/group) analyzed by flow cytometry. (E–H) BALB/c mice were immunized with low dose (0.02×106) irradiated BCR/Abl FL5.12 cells seven days prior injection of live cells. After injection of leukemic cells, mice were treated with i.p. administration of PD-1 blocking antibody (250 μg/mouse) every three days for the course of 12 days. Splenic T cells were analyzed 20 days after injection. Percentage of GFP+ cells in spleen (E) and total splenocyte count (F) on day 20. (G) Survival of FL5.12 injected mice without treatment (n=7), immunized and IgG isotype treated (n=7) and immunized and anti-PD-1 treated (n=7). (H) Glut1 expression on T cells from animals treated as in E–G was measured with flow cytometry. (I,J) Purified healthy human blood T cells were repeatedly stimulated with low-dose anti-CD3, anti-CD28, anti-CD2 coated beads for indicated times with or without rest until re-stimulation on day 21 (Supplemental Fig. 3B). Glucose uptake, pS6 on CD4+ and CD8+ T cells (I) and expression of specified markers on CD4+ T cells (J) was measured with flow cytometry. Data in C,D are mean ± s.d. * P < 0.05, ** P < 0.01 and *** P < 0.001. Differences in survival were analyzed using Log-rank (Mantel-Cox) test. Data in I are representative of 4–7 chronically stimulated and 4–6 control PBMCs samples. Data in J are mean +/− s.e.m. from 3 donors.
Although multiple mechanisms may contribute to T cell dysfunction in leukemia-bearing mice, PD-1 plays a prominent role in T cell exhaustion and is associated with poor outcome in acute and chronic B cell leukemia (9, 38). To test whether PD-1 contributes to T cell defects in this model of experimental B cell leukemia, we treated leukemic mice with anti-PD-1 blocking antibodies in vivo. Mice were immunized with a small number of irradiated FL5.12 cells seven days prior to leukemia transfer to prime for an anti-leukemic T cell response. Anti-PD-1 treatment of leukemia-bearing mice failed to significantly decrease the percentage of GFP+ leukemic cells in spleens (Fig. 2E). Anti-PD-1 treated animals showed a trend towards decreased splenocyte count (Fig. 2F) and a trend towards prolonged survival (Fig. 2G). Baseline expression of LAG3, PD-1, TIM3, CD25 and CD71 as well as activation induced T cell glucose uptake, cell size and expression of CD25 and CD75 were not different between controls and anti-PD-1 treated mice (Supplemental Fig. 2A–C). However, anti-PD-1 was sufficient to increase Glut1 expression on CD8+ T cells from leukemia-bearing animals (Figure 2H). Nevertheless, it appeared that PD-1 was, on its own, insufficient to account for all T cell defects. Consistent with this conclusion, in vitro PD-1 blockade also failed to restore the ability of T cells to properly activate (Supplemental Fig. 3A). To identify additional inhibitory molecules expressed on chronically stimulated T cells that may contribute to T cell dysfunction, we repeatedly stimulated human T cells in vitro to mimic in vivo chronic T cell activation (Supplemental Fig. 3B). Like T cells from leukemia bearing mice, chronically stimulated T cells were unable to increase 2NBDG uptake and phosphorylation of S6 upon restimulation (Fig. 2I). In addition to PD-1, chronically stimulated T cells also increased expression of the inhibitory receptor CD200R (Fig. 2J, Supplemental Fig. 3C). Thus chronic stimulation induces expression of T cell inhibitory receptors that correlate with impaired ability to upregulate mTORC1 signaling and glucose uptake upon restimulation.
Rescue of T cell Akt/mTORC1 signaling and Glut1 expression decreases leukemia-associated T cell dysfunction
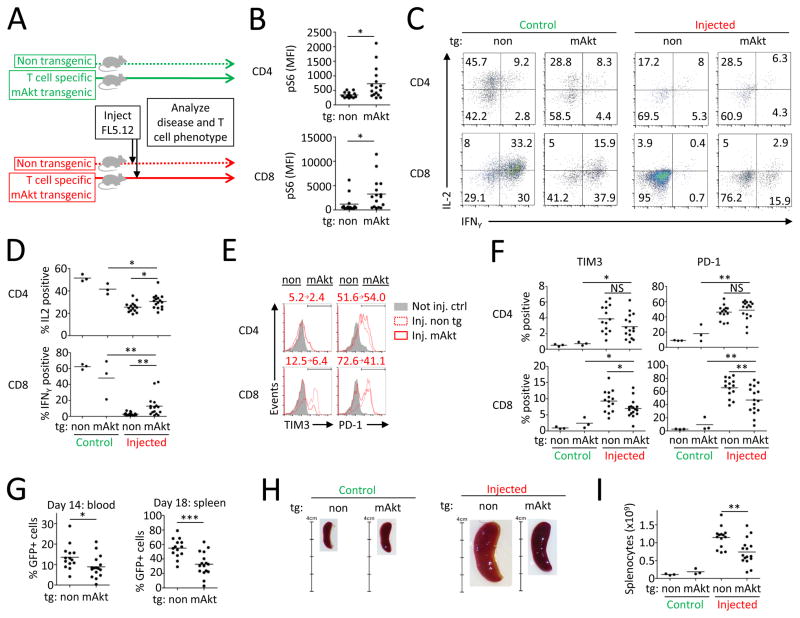
The Akt/mTORC1 pathway is critical in T cell metabolic reprogramming (21) yet mTORC1 activity was decreased in T cells in the murine leukemia model. Next, mice with T cell specific transgenic expression of constitutively active Akt (mAkt) in which T cells show increased glucose uptake and glycolysis (28) were used to test if restoration of Akt/mTORC1 signaling could improve function of T cells from leukemia-bearing animals. mAkt mice and control littermates were injected with leukemic FL5.12 cells and disease progression and T cell phenotypes were assessed (Fig. 3A). mAkt transgenic CD8+ T cells from leukemia-bearing mice had smaller cell size, lower baseline expression of CD25 and CD71 (Supplemental Fig. 3D) and higher levels of phosphorylated S6 upon stimulation (Fig. 3B). Stimulated mAkt transgenic T cells from leukemia-bearing mice showed increased ability to induce CD69, CD25, and CD71 after in vitro stimulation (Supplemental Fig. 3E) and also to produce inflammatory cytokines IL-2 and IFNγ (Fig. 3C, D). This was accompanied by decreased expression of the inhibitory receptors TIM3 and PD-1 on a subset of mAkt tg CD8+ T cells (Fig. 3E, F). Importantly, progression of leukemia was significantly delayed in mAkt transgenic mice (Fig. 3G–I) and disease burden positively correlated with T cell expression of PD-1 (Supplemental Fig. 3F). Thus inhibition of Akt/mTORC1 signaling mediates B cell leukemia-associated T cell dysfunction that both functionally impairs T cells and may prevent anti-leukemic immunity.
FIGURE 3. Rescue of T cell Akt/mTORC1 signaling decreases leukemia-induced T cell dysfunction and improves anti-leukemic immunity.
(A) Experimental design. (B) Splenocytes from FL5.12 injected mice with T cells with (n=16) or without (n=14) mAkt tg were stimulated and analyzed for S6 phosphorylation. (C,D) Production of IL-2 and IFNγ in stimulated T cells from not injected control or injected mice with or without mAkt tg. Numbers in each quadrant represent % of events. Expression of inhibitory receptors on splenic T cells (E,F) and percentage of GFP+ cells in blood at day 14 and in spleen at day 18 (G). (H,I) Spleen size at day 18. Numbers in E represent the percentage of positive cells in non tg and mAkt tg animals. * P < 0.05, ** P < 0.01 and *** P < 0.001.
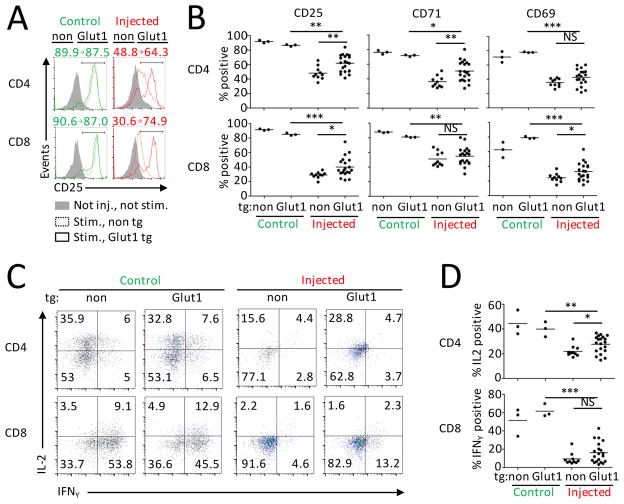
The Akt/mTORC1 pathway affects a wide range of cellular functions, including, but not limited to induction of Glut1 and glucose metabolism (28, 39). Given that T cell Glut1 expression and glucose uptake were affected in mice with B cell leukemia, we tested if leukemia-induced suppression of Glut1 expression contributed to T cell dysfunction and examined the effects of leukemia on T cells with constitutive Glut1 expression. Animals with T cell specific transgenic expression of Glut1 and increased T cell glucose uptake (20) were used as hosts for FL5.12 leukemia. Unlike with T cells with constitutively active Akt, progression of leukemia was unaffected by T cell expression of Glut1 (Supplemental Fig. 3G–I). Baseline expression of inhibitory receptors LAG3, PD-1 and TIM3 was also not changed on T cells by constitutive Glut1 expression in leukemia-bearing animals (Supplemental Fig. 3J). However, Glut1 expression was sufficient to partially rescue the ability of T cells from leukemia-bearing mice to induce CD25, CD69 and CD71 expression after anti-CD3 stimulation (Fig. 4A, B). The production of IL-2 by stimulated CD4+ T cells was also significantly increased and IFNγ production showed a trend to increase in Glut1 transgenic CD8+ T cells (Fig. 4C, D). These data demonstrate that impaired Glut1 induction contributes to T cell functional defects in B cell leukemia bearing-hosts.
FIGURE 4. Reduced Glut1 expression contributes to dysfunction of T cells from leukemia-bearing hosts.
Splenocytes from not injected mice with T cells with (n=3) or without (n=3) Glut1 tg and FL5.12-injected mice with T cells with (n=20) or without (n=10) Glut1 tg were stimulated over-night with anti-CD3. (A) Representative data of CD25 expression. Numbers represent % of positive cells in no tg and Glut1 tg animals. (B) CD25, CD71 and CD69 expression measured with flow cytometry. (C,D) Production of IL-2 and IFNγ was assessed after additional 5h stimulation with PMA/Ionomycin. Numbers in each quadrant represent % of events. * P < 0.05, ** P < 0.01 and *** P < 0.001.
T cells from human B cell leukemia patients show decreased glucose transport, glycolysis and mTORC1 activity
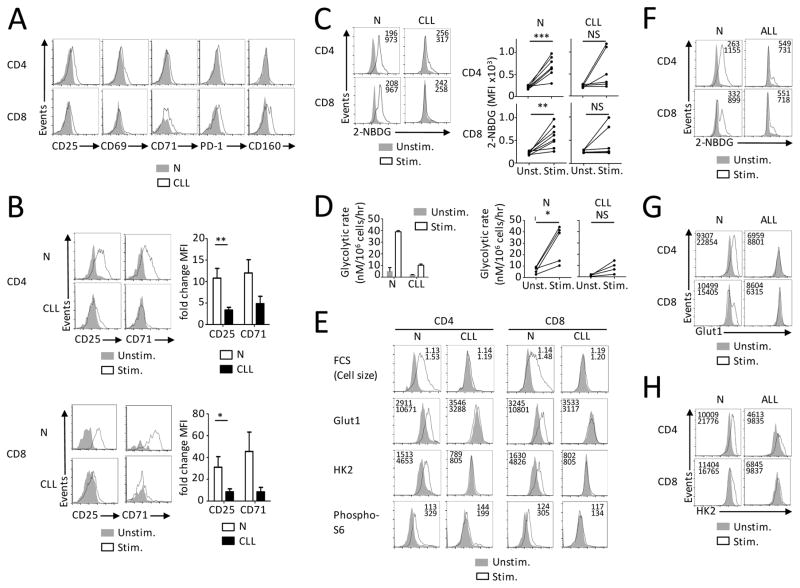
Our data in the mouse model demonstrate that B cell leukemia leads to T cell functional defects in part through metabolic suppression. To determine if similar impairments occur in patients with B cell leukemia we examined T cell activation and metabolic phenotypes of peripheral blood T cells from healthy donors and leukemia patients (described in Supplemental Table 1A). Similar to our experimental model, T cells from CLL patients had increased expression of activation markers CD25, CD69 and CD71 ex vivo. PD-1 expression was significantly increased on CD4+ T cells and CD160 was elevated on CD8+ T cells from CLL patients (Fig. 5A and Supplemental Fig. 4A, B). While the expression of PD-L1 and Gal-9 on peripheral blood mononuclear cells (PBMCs) was not increased, elevated CD200 was found on CD19+ B cells of CLL patients (Supplemental Fig. 4C, D). T cells from CLL patients also showed impaired induction of activation markers CD25 and CD71 upon in vitro stimulation (Fig. 5B). T cells from CLL patients were next analyzed to determine if the observed activation defects correlated to alterations in T cell glucose metabolism. While resting T cells from CLL patients had similar glucose uptake capacity to those from healthy donors, induction of glucose uptake (Fig. 5C) and glycolysis (Fig. 5D) was impaired in activated T cells from CLL patients. Activated T cells in healthy donors showed an increase in the expression of Glut1, HK2, and phospho-S6. T cells from CLL patients, however, showed a mixed response with notable defects in the measured metabolic parameters (Fig. 5E). Similar metabolic defects were also observed in a second cohort of CLL patients when compared to age-matched healthy control donors (Supplemental Fig. 4E). Likewise, T cells from patients with acute BCR/Abl+ B cell lymphoblastic leukemia (described in Supplemental Table 1B) also showed defects in glucose uptake, Glut1 and HK2 expression upon in vitro stimulation (Fig. 5F–H, Supplemental Fig. 4F). While no correlation of disease status and clinical parameters to the metabolic phenotype was observed, these data show that T cells in human B cell leukemia patients are metabolically impaired. Metabolic suppression of T cells is thus a mechanism of T cell dysfunction that may predispose to infections and mortality in B cell leukemia.
FIGURE 5. T cells from B cell leukemia patients show decreased glucose transport, glycolysis and mTORC1 activity.
(A) Peripheral blood mononuclear cells (PBMCs) from 5–8 healthy donors (N) and CLL patients (n=6) were analyzed for the specified markers using flow cytometry. (B) PBMCs from healthy donors (N, n=19) and CLL patients (n=16) were cultured in vitro with or without anti-CD3. After 3 days, expression of CD25 and CD71 was analyzed with flow cytometry. Fold change in MFI was calculated as a ratio of MFI values of specified markers of stimulated/not stimulated cells. (C) Glucose uptake measured with 2-NBDG incorporation and analyzed by flow cytometry on N (n=8) and CLL patients (n=6). (D) T cell glycolytic rate analyzed using samples from 5 healthy donors and 4 CLL patients. Numbers in histograms represent MFI. (E) Cell size (FSC), expression of Glut1, HK2 and phosphorylation of S6 measured with flow cytometry on T cells from N (n=8) and CLL patients (n=6). Data in B are mean ± s.e.m. Data in D are mean ± s.d. from technical triplicates. * P < 0.05, ** P < 0.01 and *** P < 0.001. (F–H) Samples form BCR/Abl+ B Cell ALL patients (n=5) or healthy controls (n=6) were stimulated as in B and glucose uptake (F), Glut1 expression (G) and HK2 expression (H) was measured by flow cytometry.
Discussion
While having a distinct biology and prognosis, both acute and chronic B cell derived leukemia share immune defects that predispose for infections and can contribute to decreased anti-tumor immunity. These immune defects are mediated in part by treatment-unrelated T cell dysfunction (7–9), but mechanisms are uncertain. Tight regulation of T cell metabolism is critical for T cell fate and function (18) and dysregulation of key metabolic pathways may contribute to T cell dysfunction. Here we show that T cells in leukemia patients and leukemia-bearing animals are metabolically suppressed and unable to efficiently increase glucose metabolism upon stimulation. This appears due to chronic in vivo stimulation leading to a phenotype similar to that described for T cell exhaustion. Importantly, these metabolic impairments are critical for the T cell dysfunction as genetic rescue of Akt signaling or glucose uptake partially restored T cell activation and function.
T cell exhaustion has been previously associated with altered cell metabolism, but this link remained largely indirect. Myc is critical for increased T cell metabolism upon activation (40) and decreased cMyc expression was reported in T cells exhausted upon chronic viral infection (41). In addition, stabilization of HIF1α has been shown to reduce T cell exhaustion (42) and overexpression of Phosphoenolpyruvate Carboxykinase 1 improved anti-tumor immunity in a mouse melanoma model (43). Recently, intra-tumoral nutrient competition was implicated as a regulator of T cell function in a mouse sarcoma model (44). The metabolic impairments of exhausted and dysfunctional cells themselves and the role of T cell metabolic dysfunction under normoxic conditions to mediate these states, however, remain unclear. Here we add to these findings and directly demonstrate that B cell leukemia induces a metabolic dysfunction of endogenous T cells that is dependent on reduced Akt/mTORC1 signaling and an inability to upregulate Glut1 and glycolysis. These metabolic defects became apparent after in vitro stimulation and required presence of leukemic cells in a co-culture model. Furthermore, these metabolic defects constituted a mechanism of T cell functional dysfunction because rescue of metabolism improved T cell function and in the case of increased Akt signaling delayed disease progression, suggesting restoration of anti-leukemic immunity. While it remains unclear to what extent the dysfunction of T cells in CLL reflects exhaustion of T cells in chronic viral infections or solid tumors, each shares features of expression of inhibitory receptors and resistance to activation. Suppression of metabolism may, thus, be a shared feature of these T cell impairments.
Despite an increase of reported leukemia-specific antigens, the source of T cell activation and impairments remain uncertain (45–47). T cells in our murine and human models appeared to be stimulated at a low level in vivo, presumably in a polyclonal antigen non-specific fashion. In addition, repeated polyclonal in vitro stimulation of naïve T cells induced a phenotype similar to in vivo stimulated T cells. The observed decrease of T cell responsiveness and impaired upregulation of metabolism may therefore result from a combination of chronic antigen stimulation and polyclonal activation. The relative contribution of antigen specific and unspecific T cell activation in vivo may vary, thus leading to heterogeneity in the phenotype of T cells from leukemic hosts. Consistent with pre-existing activation, T cells from leukemic hosts showed a trend towards increased basal expression of Glut1 and a sub-population of these T cells presented increased baseline glucose uptake. However, the T cell metabolic defects remained apparent only in the presence of leukemic cells. These results support a model in which leukemia-associated activation of T cells in vivo induces a T cell phenotype with increased expression of multiple inhibitory receptors that suppress T cell metabolism and activation.
PD-1 was upregulated on a subset of T cells from leukemic mice and increased expression of PD-L1 was detected on cells in leukemic micro-environment. PD-1 ligation inhibits PI3K/Akt/mTOR signaling and reduces Myc expression (27, 34). These pathways are critical to allow activated T cells to undergo metabolic reprogramming necessary for effector function and their inhibition would prevent T cell activation and function (40, 48, 49). In vivo blockade of PD-1 alone was insufficient to completely restore T cell function and induce anti-tumor immunity in our murine B cell leukemia model. Nevertheless, anti-PD-1 therapy increased Glut1 expression on CD8+ T cells from leukemic hosts, suggesting that PD-1 signaling contributed to leukemia-induced T cell dysfunction by metabolic suppression. Blockade of PD-1/PD-L1 interaction has proven promising to re-activate exhausted T cells in some solid tumors (50) and more recently in a mouse model of CLL (17, 51). PD-1 expression is associated with poor outcome in CLL (9) and PD-1 blockade alone or in combination with other therapies may therefore provide an approach for CLL patients.
We found that T cells from leukemic hosts expressed inhibitory receptors whose ligands were expressed in leukemic micro-environment. CD200 for example was highly upregulated on CLL cells and CD200/CD200R signaling has been reported to suppress T cell effector functions by indoleamine 2,3-dioxygenase (IDO) induction. Furthermore, CD200 and PD-1L can induce Treg cells (36, 37, 52). We observed increased percentage of FOXP3+ CD4+ Treg cells in spleens from leukemia-bearing animals. Treg cells are able to suppress the function of effector T cells and CD200 signaling might have contributed to metabolic dysfunction of non-Treg cells in B cell leukemia by Treg induction. Furthermore, CD200 blockade can increase antigen-specific T cell responses in human CLL (52). Blockade of multiple immune checkpoints may therefore be necessary to induce sufficient anti-tumor immunity in B cell leukemia. In addition to inhibition of T cell inhibitory pathways, direct metabolic modulation of T cells in vivo is also possible, but may impact leukemic cells to potentially complicate therapy. It may be more feasible to modulate T cell metabolism ex vivo. In this approach, modulation of T cell metabolic pathways or adjustment of in vitro culture conditions prior to cellular therapies such as treatment with Chimeric Antigen Receptor T cells may decrease the observed T cell exhaustion (53) and increase efficiency.
Together our studies show that alteration of key T cell metabolic pathways critical for T cell activation and function such as Akt/mTORC1 and those involved in glucose uptake and glycolysis is an important part of T cell dysregulation in B cell leukemia. It is likely that similar metabolic impairments contribute to an altered T cell phenotype in other settings, including chronic viral infection. As these metabolic pathways are essential for proper T cell responses in both protective and anti-leukemic immunity, modulation of T cell metabolism may represent a new therapeutic avenue for leukemia patients.
Supplementary Material
Acknowledgments
We would like to thank members of the Rathmell lab for support and helpful discussions. This paper is dedicated to the memory of Dr. Jozef Šiška.
Funding: This work was supported by grants from the Wade F. B. Thompson/Cancer Research Institute CLIP Grant (J.C.R.), the Duke Cancer Institute (J.C.R.), the National Institutes of Health R01DK105550 (J.C.R.) and F31CA183529 (R.J.K), the German Research Foundation (Deutsche Forschungsgemeinschaft; P.J.S.), the Biomarker Factory (J.B.W.), the VA Research Service (J.B.W.), VENI grant (Netherlands Organization for Scientific Research; G.J.W.vdW.), Marie Curie Career Integration Grant (G.J.W.vdW) and VIDI grant 91715337 (Netherlands Organization for Scientific Research; A.P.K.).
Abbreviations used in this article
- mTORC1
mammalian target of rapamycin complex 1
- HK2
Hexokinase 2
- Treg
regulatory T cells
- PI3K
Phosphatidylinositide 3-kinase
- tg
transgenic
- mAkt
myristoylated Akt
- IFNγ
interferon gamma
- CLL
B cell chronic lymphocytic leukemia
- CMV
cytomegalovirus
- PBMCs
peripheral blood mononuclear cells
- TGFβ
transforming growth factor beta
Footnotes
Conflict of Interests: The authors have no conflicts of interest to declare
References
- 1.O’Connor D, Bate J, Wade R, Clack R, Dhir S, Hough R, Vora A, Goulden N, Samarasinghe S. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood. 2014;124:1056–1061. doi: 10.1182/blood-2014-03-560847. [DOI] [PubMed] [Google Scholar]
- 2.Melchardt T, Weiss L, Greil R, Egle A. Viral infections and their management in patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2013;54:1602–1613. doi: 10.3109/10428194.2012.755178. [DOI] [PubMed] [Google Scholar]
- 3.Thijssen R, Ter Burg J, van Bochove GG, de Rooij MF, Kuil A, Jansen MH, Kuijpers TW, Baars JW, Virone-Oddos A, Spaargaren M, Egile C, van Oers MH, Eldering E, Kersten MJ, Kater AP. The pan phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor SAR245409 (voxtalisib/XL765) blocks survival, adhesion and proliferation of primary chronic lymphocytic leukemia cells. Leukemia. 2016;30:337–345. doi: 10.1038/leu.2015.241. [DOI] [PubMed] [Google Scholar]
- 4.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, Ramsay AG, Gribben JG. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopoulos P, Pfeifer D, Bartholome K, Follo M, Timmer J, Fisch P, Veelken H. Definition and characterization of the systemic T-cell dysregulation in untreated indolent B-cell lymphoma and very early CLL. Blood. 2011;117:3836–3846. doi: 10.1182/blood-2010-07-299321. [DOI] [PubMed] [Google Scholar]
- 7.Yotnda P, Mintz P, Grigoriadou K, Lemonnier F, Vilmer E, Langlade-Demoyen P. Analysis of T-cell defects in the specific immune response against acute lymphoblastic leukemia cells. Experimental hematology. 1999;27:1375–1383. doi: 10.1016/s0301-472x(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 8.Brusa D, Serra S, Coscia M, Rossi D, D’Arena G, Laurenti L, Jaksic O, Fedele G, Inghirami G, Gaidano G, Malavasi F, Deaglio S. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–963. doi: 10.3324/haematol.2012.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:678–687. doi: 10.1158/1078-0432.CCR-11-2630. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. Journal of immunology. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 13.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends in immunology. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossmann ED, Jeddi-Tehrani M, Osterborg A, Mellstedt H. T-cell signaling and costimulatory molecules in B-chronic lymphocytic leukemia (B-CLL): an increased abnormal expression by advancing stage. Leukemia. 2003;17:2252–2254. doi: 10.1038/sj.leu.2403100. [DOI] [PubMed] [Google Scholar]
- 15.Van den Hove LE, Vandenberghe P, Van Gool SW, Ceuppens JL, Demuynck H, Verhoef GE, Boogaerts MA. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: evidence for systemic activation of the T cell compartment. Leuk Res. 1998;22:175–184. doi: 10.1016/s0145-2126(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 16.Yang ZZ, Grote DM, Xiu B, Ziesmer SC, Price-Troska TL, Hodge LS, Yates DM, Novak AJ, Ansell SM. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin’s lymphoma. Leukemia. 2014;28:1872–1884. doi: 10.1038/leu.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClanahan F, Hanna B, Miller S, Clear AJ, Lichter P, Gribben JG, Seiffert M. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood. 2015;126:203–211. doi: 10.1182/blood-2015-01-622936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annual review of immunology. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Molecular biology of the cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. Journal of immunology. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends in immunology. 2015;36:13–20. doi: 10.1016/j.it.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macintyre AN, V, Gerriets A, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, Rathmell JC. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalek RD, V, Gerriets A, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of immunology. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. Journal of immunology. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nature communications. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. European journal of immunology. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Altman BJ, Coloff JL, Herman CE, Jacobs SR, Wieman HL, Wofford JA, Dimascio LN, Ilkayeva O, Kelekar A, Reya T, Rathmell JC. Glycogen synthase kinase 3alpha and 3beta mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKearn JP, McCubrey J, Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason EF, Zhao Y, Goraksha-Hicks P, Coloff JL, Gannon H, Jones SN, Rathmell JC. Aerobic glycolysis suppresses p53 activity to provide selective protection from apoptosis upon loss of growth signals or inhibition of BCR-Abl. Cancer Res. 2010;70:8066–8076. doi: 10.1158/0008-5472.CAN-10-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ L. International Workshop on Chronic Lymphocytic. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. Journal of biochemical and biophysical methods. 2005;64:207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Patsoukis N, Li L, Sari D, Petkova V, Boussiotis VA. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol Cell Biol. 2013;33:3091–3098. doi: 10.1128/MCB.00319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. Journal of immunology. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, Kline J. PD-1 regulates extrathymic regulatory T-cell differentiation. European journal of immunology. 2014;44:2603–2616. doi: 10.1002/eji.201344423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francisco LM, V, Salinas H, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Liu S, Wang L, Zhang W, Ji Y, Ma X. Clinical significance of B7-H1 (PD-L1) expression in human acute leukemia. Cancer Biol Ther. 2008;7:622–627. doi: 10.4161/cbt.7.5.5689. [DOI] [PubMed] [Google Scholar]
- 39.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nature immunology. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, Kleinstein SH, Abel ED, Insogna KL, Feske S, Locasale JW, Bosenberg MW, Rathmell JC, Kaech SM. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowalewski DJ, Schuster H, Backert L, Berlin C, Kahn S, Kanz L, Salih HR, Rammensee HG, Stevanovic S, Stickel JS. HLA ligandome analysis identifies the underlying specificities of spontaneous antileukemia immune responses in chronic lymphocytic leukemia (CLL) Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E166–175. doi: 10.1073/pnas.1416389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajasagi M, Shukla SA, Fritsch EF, Keskin DB, DeLuca D, Carmona E, Zhang W, Sougnez C, Cibulskis K, Sidney J, Stevenson K, Ritz J, Neuberg D, Brusic V, Gabriel S, Lander ES, Getz G, Hacohen N, Wu CJ. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kessler JH, Melief CJ. Identification of T-cell epitopes for cancer immunotherapy. Leukemia. 2007;21:1859–1874. doi: 10.1038/sj.leu.2404787. [DOI] [PubMed] [Google Scholar]
- 48.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends in immunology. 2015;36:257–264. doi: 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kater AP, van der Windt GJ. PD-L1 blockade: rejuvenating T cells in CLL. Blood. 2015;126:126–128. doi: 10.1182/blood-2015-05-638338. [DOI] [PubMed] [Google Scholar]
- 52.Pallasch CP, Ulbrich S, Brinker R, Hallek M, Uger RA, Wendtner CM. Disruption of T cell suppression in chronic lymphocytic leukemia by CD200 blockade. Leuk Res. 2009;33:460–464. doi: 10.1016/j.leukres.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, Patterson GH, Fry TJ, Orentas RJ, Mackall CL. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature medicine. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.