Abstract
Epithelial-mesenchymal transition (EMT) describes the global process by which stationary epithelial cells undergo phenotypic changes, including loss of cell-cell adhesion and apical-basal polarity, and acquire mesenchymal characteristics which confer migratory capacity. EMT and its converse, MET (mesenchymal-to-epithelial transition), are integral stages of many physiologic processes, and as such are tightly coordinated by a host of molecular regulators. Converging lines of evidence have identified EMT as a component of cutaneous wound healing, during which otherwise stationary keratinocytes - the resident skin epithelial cells - migrate across the wound bed to restore the epidermal barrier. Moreover, EMT also plays a role in the development of scarring and fibrosis, as the matrix-producing myofibroblast arises from cells of epithelial lineage in response to injury but is pathologically sustained instead of undergoing MET or apoptosis. In this review, we summarize the role of EMT in physiologic repair and pathologic fibrosis of tissues and organs. We conclude that further investigation into the contribution of EMT to the impaired repair of fibrotic wounds may identify components of EMT signaling as common therapeutic targets for impaired healing in many tissues.
INTRODUCTION
Epithelial-mesenchymal transition (EMT) is a process during which epithelial cells gradually transform into mesenchymal-like cells and lose their epithelial functionality and characteristics. Converging lines of evidence suggest that EMT plays a role in both physiologic and pathologic healing. In this Review, we summarize findings from animal and human wound healing models that support the importance of proper execution of EMT in achieving successful tissue repair following injury. For instance, during cutaneous wound healing epidermal keratinocytes undergo EMT by losing their adherent epithelial phenotype to become motile cells with a mesenchymal phenotype which migrate across the wound bed (Yan, et al., 2010). We discuss several growth factors common to both wound healing and EMT, such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF) and transforming growth factor-beta (TGFβ), and highlight shared signaling pathways.
While EMT is necessary for proper re-epithelialization and extracellular matrix deposition, uncontrolled continued transition from epithelial cells to myofibroblasts may result in fibrosis. We discuss the role of EMT in generating myofibroblasts from resident epithelial cells during the maturation phase of wound healing. We summarize evidence that sustained EMT is a key mechanism underlying the fibrotic pathology of multiple organs including the skin. The role of EMT in pathophysiology of renal, pulmonary, cardiac and liver fibrosis, cutaneous scleroderma, and impaired wound healing are also discussed.
GLOBAL FEATURES OF EMT
EMT is often divided by biological context into three subtypes: Type I, which occurs during embryogenesis; Type II, occurring during tissue repair; and Type III, which occurs during the metastatic spread of cancer. The three types of EMT have a shared outcome: the production of motile cells with a mesenchymal phenotype from otherwise classically adherent epithelial cells with apical-basal polarity (Kalluri and Neilson, 2003). However, in contrast to Types I and III, Type II EMT is instigated exclusively by damage and inflammation (Volk, et al., 2013).
The first step of EMT is the loss of epithelial cell markers, one of the most notable of which is decreased expression of E-cadherin (Whiteman, et al., 2008). E-cadherin is responsible for maintaining the epithelial cells’ lateral contacts via adherens junctions, as well as cell adhesion and relative immobility in the tissue (Huang, et al., 2012, Moreno-Bueno, et al., 2008, Qin, et al., 2005). E-cadherin downregulation is also mediated through upregulation of vimentin, an intermediate filament that decreases E-cadherin trafficking to the cell surface (Mendez, et al., 2010). The cell then progresses towards a mesenchymal phenotype by gaining mesenchymal markers and capabilities (Lee, et al., 2006). This change is orchestrated by temporally regulated expression of proteins including neural cadherin (N-cadherin), vimentin, integrin, fibronectin, and matrix metalloproteinases (MMPs) (Huang, Guilford and Thiery, 2012, Thiery and Sleeman, 2006, Wheelock, et al., 2008). Integrins that \ interact with extracellular matrix (ECM) components such as fibronectin are then upregulated to increase motility (Maschler, et al., 2005, Yang, et al., 2009). A driving force behind this motility is the loss of the polarized cytoskeleton in epithelial cells, and the development of lamellipodia in the advancing edge of the transitioning mesenchymal cells (Takenawa and Suetsugu, 2007). It is noteworthy that EMT process may not always be a complete. In some instances, cells may exist along a gradient where incomplete transition occurs, and both epithelial and mesenchymal characteristics are exhibited by the same cell (Jordan, et al., 2011).
EMT IN PHYSIOLOGIC TISSUE REPAIR
Wound healing exhibits EMT-like features
Converging lines of evidence indicate that EMT is an essential component of physiologic tissue repair. The majority of studies have been conducted in models of cutaneous wound healing. Wound healing consists of several overlapping phases that involve an injury-induced inflammatory response which is associated with cellular proliferation, migration, and ECM remodeling (Eming, et al., 2014, Martin, 1997). Of these processes, the one most reminiscent of EMT is the process of re-epithelialization, which has been termed “partial EMT” (Arnoux, et al., 2005). As discussed above, a hallmark of EMT is cell-cell dissociation and acquisition of motility, and during re-epithelialization keratinocytes at the wound edge lose their intercellular adhesions and migrate across the wound (Coulombe, 2003). Specifically, these keratinocytes undergo changes in junctional complexes including reduction in desmosomes and adherens junctions, disruption of intermediate filaments, and cytoskeletal reorganization which results in the creation of intercellular gaps (Baum and Arpey, 2005, Santoro and Gaudino, 2005). These changes enable the keratinocytes to shift morphologically from cuboidal and stationary to flattened and migratory, with extended lamellipodia (Baum and Arpey, 2005, Santoro and Gaudino, 2005). There is also evidence that myofibroblasts, the key players in the remodeling and maturation phase of wound healing, are derived from resident epithelial cells that have transformed through EMT to synthesize ECM components and to contract the wound bed, enabling approximation of the injured edges (Iwano, et al., 2002, Radisky, et al., 2007, Wynn and Ramalingam, 2012).
EMT is implicated in animal and human models of cutaneous wound healing
Evidence from in vitro, in vivo, and ex vivo animal and human models support the importance of proper execution of EMT in achieving successful wound repair following cutaneous injury. To start with, the EMT transcription factor Slug has been implicated in the process of re-epithelialization in numerous studies. Healing of excisional wounds is impaired in Slug knockout mice almost twofold in comparison to wild-type controls (Hudson, et al., 2009), and epidermal keratinocytes from these mice display defects in migration (Savagner, et al., 2005). In ex vivo skin explants from Slug null mice, epithelial cell outgrowth is also severely impaired, again indicating compromised motility (Savagner, Kusewitt, Carver, Magnino, Choi, Gridley and Hudson, 2005) (Kusewitt, et al., 2009). Indeed, Slug expression is elevated in wild-type keratinocytes at the edges of murine wounds in vivo (Shirley, et al., 2010) (Savagner, Kusewitt, Carver, Magnino, Choi, Gridley and Hudson, 2005), and its expression specifically increases in the actively migrating mouse keratinocytes (Savagner, Kusewitt, Carver, Magnino, Choi, Gridley and Hudson, 2005).
Mechanistically, Slug regulates keratinocyte motility during re-epithelialization by repressing E-cadherin, leading to decreased cell-cell adhesion (Savagner, 2001). It also drives intercellular desmosomal disruption at the wound edge (Savagner, Kusewitt, Carver, Magnino, Choi, Gridley and Hudson, 2005). Finally, the epidermal growth factor receptor (EGFR) signaling pathway that is integral to re-epithelialization in physiologic wound healing may be the master regulator of EMT/Slug-mediated effects, since EGFR ligands stimulate the expression of Slug as well as subsequent migration in keratinocytes (Kusewitt, Choi, Newkirk, Leroy, Li, Chavez and Hudson, 2009) in a process that is mediated by Erk5 (Arnoux, et al., 2008). Indeed, in the absence of Slug, EGFR ligands are unable to stimulate migration of skin explants in the ex vivo model of physiologic re-epithelialization (Kusewitt, Choi, Newkirk, Leroy, Li, Chavez and Hudson, 2009).
Work in additional mammalian models provides further evidence for EMT involvement in skin repair. Treatment of rat mucosal keratinocytes with EGFR ligands as well as inflammatory cytokines TGFβ or interleukin 1 beta (IL1β) induces EMT-associated metalloproteinases MMP9 and MMP13 as well as EMT-like changes in cell morphology (Lyons, et al., 1993). The N-acetylglucosaminyltransferase V transgenic (GnT-V Tg) mouse, which features aberrant structural modifications of oligosaccharides, carries an enhanced EMT-like phenotype which culminates in rapid re-epithelialization in vivo, in part due to differential glycosylation of EGFR and subsequent amplification of signaling which leads to increased migration (Terao, et al., 2011). Specifically, wounded GnT-V keratinocytes exhibit spindle-like morphology, increased expression of EMT factors N-cadherin, Snail and Twist, and enhanced migration (Terao, Ishikawa, Nakahara, Kimura, Kato, Moriwaki, Kamada, Murota, Taniguchi, Katayama and Miyoshi, 2011). Foxn1, a potent mammalian wound healing factor, also appears to be involved in EMT-driven re-epithelialization during repair, as evidenced by studies in Foxn1 transgenic mice. In these mice, the induction of EMT post-wounding was demonstrated though the upregulation of EMT transcriptional regulator Snail1, increased MMP9 expression, presence of vimentin+/E-cadherin+ cells, and migratory keratinocytes at the wound edge expressed Foxn1 which co-localized with Snail (Gawronska-Kozak, et al., 2016). Finally, zebrafish keratocytes in explant culture, which serve as a well-studied model of epithelial wound healing, display evidence of EMT (McDonald, et al., 2013). During injury-triggered migration, keratocytes feature loss of epithelial keratins and E-cadherin accompanied by gain of mesenchymal markers vimentin and N-cadherin. Moreover, explanted zebrafish keratocytes exhibit EMT-like morphologic changes including actin cytoskeletal rearrangements, disassembly of cellular sheets, and flattened cells. Interestingly, cell motility in this model appears to be driven in part by TGFβ1 (Tan, et al., 2011) which is a known trigger of EMT.
In in vitro models of human wound healing, immortalized HaCaT keratinocytes with forced overexpression of the EMT transcription factor Slug feature enhanced migration and disruption of desmosomes at the wound margin, recapitulating its effects in wounded skin of animal models in vivo (Savagner, Kusewitt, Carver, Magnino, Choi, Gridley and Hudson, 2005). Similarly, antimicrobial peptides shown to enhance wound healing concurrently induce Slug at the edge of wounded HaCaTs (Carretero, et al., 2008). Heparin-binding EGF (HB-EGF), a keratinocyte-expressed ligand which activates EGFR during human wound healing (Mathay, et al., 2008, McCarthy, et al., 1996, Stoll, et al., 1997), triggers a migratory phenotype that is reminiscent of EMT. Specifically, expression of HB-EGF in human keratinocytes decreases epithelial keratins and E-cadherin, increases vimentin expression, and increases EMT factors SNAIL1 and ZEB1. HB-EGF also increases COX2 and MMP1, which are additional markers of cellular motility (Stoll, et al., 2012). But perhaps the most compelling evidence for the involvement of EMT in human cutaneous wound healing originates from a study by Yan et al (Yan, Grimm, Garner, Qin, Travis, Tan and Han, 2010) which demonstrated what the authors termed “partial EMT” in wound healing in vitro, ex vivo and in vivo. Basal keratinocytes in the migrating tongue of re-epithelializing human acute wounds gained expression of mesenchymal markers fibroblast-specific protein 1 (FSP1) and/or vimentin, while the basement membrane zone displayed collagen disassembly, reflecting EMT-associated degradation of the ECM. Furthermore, treatment of ex vivo human skin with inflammatory cytokines tumor necrosis factor- alpha (TNFα) and TGFβ induced an EMT-positive cell population. Primary keratinocytes treated similarly displayed morphologic cellular elongation as well as an enhanced migratory phenotype which was reversible following removal of cytokine stimuli. As such, injury-inducible mobilization of epithelial cells involving TNFα and bone morphogenetic protein (BMP)-2 produced a mesenchymal phenotype in migrating keratinocytes (Yan, Grimm, Garner, Qin, Travis, Tan and Han, 2010).
Role of EMT in extra-cutaneous organ repair
There is additional evidence for EMT occurring during repair of organs other than the skin. During in vitro healing of a breast (mammary) epithelial cell line, time-lapse microscopy indicated that EMT-associated vimentin was expressed in a migration-dependent fashion, such that vimentin was exclusively induced in actively migrating cells at the leading wound edge, which was accompanied by actin filament reorganization. Vimentin expression subsequently disappeared once wound closure was achieved (Gilles, et al., 1999). Similarly, in a murine model of lacrimal gland injury, inflammation induced by interleukin-1 (IL-1) injection triggered the generation and migration of cells with mesenchymal features to the site of injury, which subsequently reverted to an epithelial phenotype once repair was complete (You, et al., 2012). These cells initially expressed EMT markers Snail1 and vimentin during the repair phase, the levels of which decreased after injury resolution, indicating a reversible or “partial” EMT. Finally, EMT is a key feature of cardiac development during embryogenesis, and accumulating evidence in zebrafish and other models of myocardial injury indicates that a subpopulation of epicardial cells undergo EMT to regenerate the damaged epithelial cover and help establish new vasculature (Lepilina, et al., 2006) (Krainock, et al., 2016).
Wound healing and EMT share central signaling pathways
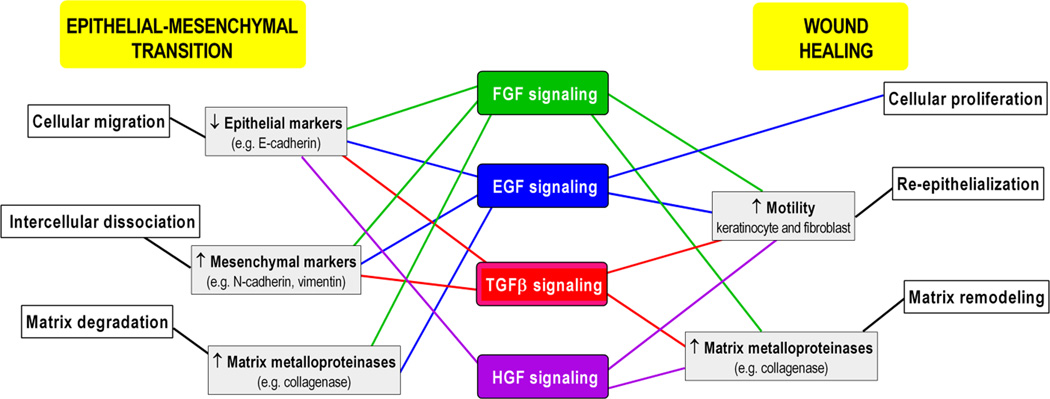
It is noteworthy that a complex signaling network involving numerous growth factors activated during wound healing are also involved in the initiation and regulation of the EMT, supporting a global role for EMT in epithelial barrier restoration following injury (Figure 1). The common growth factors indispensable for both processes include FGF, EGF, HGF and TGFβ (Akhurst and Derynck, 2001, Camenisch, et al., 2002, Jechlinger, et al., 2006, Kim, et al., 2007, Murillo, et al., 2005, Nawshad and Hay, 2003). FGF, EGF, and HGF function as ligands for the corresponding receptors, tyrosine kinase transmembrane proteins, resulting in their dimerization and autophosphorylation, phosphorylation of the downstream target proteins, and activation of the signaling cascades (Lemmon and Schlessinger, 2010, Tsai and Yang, 2013). Thus, ERK MAPK, p38 MAPK, and JNK are among the activated pathways that ultimately upregulate EMT transcription factors such as SNAIL, Slug, and ZEB (Tsai and Yang, 2013) on one hand, while triggering wound healing processes on the other (Castilho, et al., 2013, Zhang, et al., 2015).
Fig. 1.
Common growth factor signals initiate and regulate essential EMT and wound-healing processes. Please refer to text for supporting references. FGF, fibroblast growth factor; EGF, epidermal growth factor; TGFβ, transforming growth factor beta; HGF, hepatocyte growth factor.
FGF signaling
The FGF family comprises 23 members, while the three crucial FGFs for the wound healing process include FGF-2, FGF-7, and FGF-10 (Golinko, et al., 2009). FGF-2, or basic FGF, is increased in the acute wound and plays a role in granulation tissue formation, epithelialization, and tissue remodeling (Powers, et al., 2000). In vitro studies have shown that activation of the FGF receptor by FGF-2 increases keratinocyte and fibroblast motility (Di Vita, et al., 2006, Sogabe, et al., 2006), and stimulates fibroblasts to produce collagenase (Sasaki, 1992). The FGF family is also induced during EMT (Smith and Bhowmick, 2016), with the role to ensure that epithelial cells adopt a mesenchymal phenotype through classic effects such as down-regulation of E-cadherin and catenins and induction of mesenchymal MMPs (Ciruna, et al., 1997, Strutz, et al., 2002). In particular, FGF-2 is important in repair-associated EMT (Ciruna, Schwartz, Harpal, Yamaguchi and Rossant, 1997, Sun, et al., 1999). . Other FGF family members (e.g. FGF-1) instigate EMT in carcinomas, prompting an increase in the EMT transcription factor Slug, downregulation of desmosomal components and upregulation of MMPs and integrins, all of which are essential for cell motility (Billottet, et al., 2008, Savagner, et al., 1997, Valles, et al., 1996).
EGF signaling
The EGF family represents the best-characterized growth factor family in wound healing and includes a wide variety of ligands such as EGF, HB-EGF, transforming growth factor-alpha (TGFα), Cripto-1, epiregulin, amphiregulin, betacellulin, epigen, and neuregulins (NRG) 1–6 (Barrientos, et al., 2014, Barrientos, et al., 2008). Ultimately, EGF signaling leads to the activation of a number of converging signaling pathways promoting keratinocyte migration and proliferation (Omenetti, et al., 2008). EGF also aids to accomplish EMT by down- regulating E-cadherin via E-cadherin internalization, upregulating SNAIL1 and/or TWIST, and increasing cell motility through MMP-directed ECM degradation (Ahmed, et al., 2006, Lo, et al., 2007, Lu, et al., 2003). In murine mammary epithelial cell tumors, upregulation of Cripto1, an EGF family member, results in enhanced mesenchymal characteristics, such as increased expression of N-cadherin, vimentin, and Snail1 expression (Rangel, et al., 2012, Strizzi, et al., 2004, Tao, et al., 2005).
HGF signaling
HGF signaling is an additional example of the wound healing – EMT crosstalk. HGF, mainly produced by fibroblasts, exerts its function by binding to its tyrosine kinase receptor c-Met (mesenchymal epithelial transition factor, or HGFR), which is expressed on the surface of keratinocytes (Toyoda, et al., 2001). Both HGF and c-Met are upregulated during wound healing and promote granulation tissue formation and neoangiogenesis (Toyoda, Takayama, Horiguchi, Otsuka, Fukusato, Merlino, Takagi and Mori, 2001, Wang, et al., 2009, Yoshida, et al., 2003). Furthermore, c-Met plays an important role in re-epithelialization through activation of PI3K/AKT, ERK1/2, Gab1 (Grb2-associated-binding protein 1) and PAK1/2 (p21-activated protein kinase) signaling (Chmielowiec, et al., 2007). HGF and its receptor also clearly induce various changes in the EMT process, depending on the specific cell type expressing c-Met (Grotegut, et al., 2006, Savagner, Yamada and Thiery, 1997). To begin with, HGF can regulate master EMT transcription factor SNAIL1 (which decreases E-cadherin)and Slug (which decreases desmoplakins) aiding in the breakdown of intercellular adhesions (Grotegut, von Schweinitz, Christofori and Lehembre, 2006, Savagner, Yamada and Thiery, 1997). Additionally, the c-Met-PI3K/AKT pathway influences cell cycle, proliferation and quiescence (King, et al., 2015), and PI3K-activated mTORC2 is one of the driving factors for the phenotypic transition in EMT, while mTORC1 encourages cell growth and movement (Lamouille, et al., 2012, Lamouille and Derynck, 2007). Since one of AKT’s roles is to phosphorylate and inactivate GSK3β, which itself is an inhibitor of SNAIL1 expression, inhibition of AKT can cause down regulation of SNAIL activity in the cell and impede EMT (Lamouille, Connolly, Smyth, Akhurst and Derynck, 2012, Zhou, et al., 2004). The resultant decrease in MMP production and non-inhibited production of E-cadherin makes EMT and subsequent movement difficult for the cell to achieve (Lamouille, Connolly, Smyth, Akhurst and Derynck, 2012).
TGFβ signaling in wound healing, EMT, and fibrosis
The TGFβ pathway is well studied not only in wound healing (Ramirez, et al., 2014) but also in all three types of EMT (Akhurst and Derynck, 2001, Camenisch, Molin, Person, Runyan, Gittenberger-de Groot, McDonald and Klewer, 2002, Nawshad and Hay, 2003). TGFβ progresses via two pathways, SMAD-dependent and SMAD-independent (Xu, et al., 2000). In SMAD dependent pathways, the TGFβ cell surface receptors (known as TGFβ receptors type II) are activated by ligand and phosphorylate the transmembrane kinases (TGFβ receptor type I), which then forms a SMAD complex; this complex can enter the nucleus, subsequently activating or inhibiting transcription factors important for either wound healing or EMT (Derynck and Zhang, 2003, Ramirez, Patel and Pastar, 2014). In wound healing, TGFβ1 play important roles in inflammation, angiogenesis, re-epithelialization, and connective tissue regeneration (Ramirez, Patel and Pastar, 2014). TGFβ and SMAD complexes induce SNAIL1 expression, and themselves are potent downregulators of E-cadherin, occludin, and other epithelial phenotypic markers, while promoting mesenchymal markers such as vimentin and N-cadherin (Vincent, et al., 2009). SMAD3-SMAD4 complexes can also activate TWIST and ZEB transcription factors, via the MAPK signaling route, one of the SMAD-independent pathways (Javelaud and Mauviel, 2005). Another major SMAD-independent pathway is the PI3K/AKT pathway, whose importance in both EMT and wound healing is discussed previously.
EMT IN SCARRING AND FIBROSIS
EMT-derived myofibroblasts, TGFβ, and fibrosis
During physiologic repair, tissue integrity must be restored not only through re-epithelialization but also through formation of a stress-resistant scar. The cellular orchestrator of this remodeling process is the contractile myofibroblast, which secretes large amounts of ECM proteins and aids in the mechanical closure of the wound (Gabbiani, et al., 1971, Hinz and Gabbiani, 2003). In normal wound healing, many myofibroblasts undergo apoptosis and disappear once re-epithelialization is complete (Desmouliere, et al., 1995, Gabbiani, 2003). However, pathologically prolonged myofibroblast activity results in fibrogenesis. Indeed, persistent myofibroblast activation is a shared feature of fibrotic diseases. As such, the dysregulation of injury-triggered EMT is believed to contribute to fibrosis of multiple organs.
Though the myofibroblast can be derived from a variety of sources (Abe, et al., 2001, Direkze, et al., 2003, Ebihara, et al., 2006, Frid, et al., 2002, Higashiyama, et al., 2011, Wynn and Ramalingam, 2012), a large body of evidence supports that a proportion of them arise through EMT during organ fibrosis. Moreover, TGFβ1, a critical regulator of EMT signaling as well as physiologic wound healing (as discussed above), is also the major driver of fibrosis (Border and Noble, 1994, Roberts, et al., 1986), in part through its role in sustaining myofibroblast activation (Desmouliere, et al., 1993, Gabbiani, 2003, Hong, et al., 2007, Ronnov-Jessen and Petersen, 1993, Serini and Gabbiani, 1999). This section focuses on evidence implicating EMT in fibrogenesis of different tissues, which arise as a pathological response to injury.
Renal fibrosis
Progressive chronic kidney disease characterized by interstitial fibrosis can lead to tubular atrophy, loss of kidney function and end-stage renal failure (Liu, 2011). Numerous studies have provided evidence that EMT-derived myofibroblasts originating from tubular epithelia contribute to renal fibrosis. These studies have used animal models, human kidney biopsies, staining techniques for epithelial and fibroblast cell lineage markers, lineage tags and activation of various transcriptional signals known to activate the EMT program (Higgins, et al., 2007, Humphreys, et al., 2010, Inoue, et al., 2009, Iwano, Plieth, Danoff, Xue, Okada and Neilson, 2002, Nishitani, et al., 2005, Rastaldi, et al., 2002, Strutz, Zeisberg, Ziyadeh, Yang, Kalluri, Muller and Neilson, 2002, Zeisberg, et al., 2003). Though conflicting at times, a series of genetic-lineage tracking and fate-mapping studies have provided support for existence of EMT-derived myofibroblasts in renal fibrosis (Humphreys, Lin, Kobayashi, Hudson, Nowlin, Bonventre, Valerius, McMahon and Duffield, 2010). In one experimental murine model, fibroblasts expressing the mesenchymal EMT marker FSP1 were shown to derive from both the bone marrow and local EMT during renal fibrogenesis (Iwano, Plieth, Danoff, Xue, Okada and Neilson, 2002). In vivo evidence for EMT in renal fibrosis has also been reported in human biopsy studies (Inoue, Okada, Takenaka, Watanabe and Suzuki, 2009, Nishitani, Iwano, Yamaguchi, Harada, Nakatani, Akai, Nishino, Shiiki, Kanauchi, Saito and Neilson, 2005, Rastaldi, Ferrario, Giardino, Dell’Antonio, Grillo, Grillo, Strutz, Muller, Colasanti and D’Amico, 2002). In a patient with fibrosis-inducing obstructive nephropathy, obstructed tubular epithelial cells expressed FSP1 (Okada, et al., 1997), and some adopted an EMT-like fibroblast morphology (Inoue, Okada, Takenaka, Watanabe and Suzuki, 2009, Nishitani, Iwano, Yamaguchi, Harada, Nakatani, Akai, Nishino, Shiiki, Kanauchi, Saito and Neilson, 2005). FSP1 has also been shown to be a prognostic marker in renal fibrosis in humans (Nishitani, Iwano, Yamaguchi, Harada, Nakatani, Akai, Nishino, Shiiki, Kanauchi, Saito and Neilson, 2005). Another study of 133 biopsies from various renal fibrosis conditions demonstrated that tubular epithelia cells produced a variety of ECM proteins characteristic of a mesenchymal phenotype, the levels of which correlated clinically with elevated serum creatinine levels and indices of renal dysfunction as well as histologic extent of interstitial fibrotic damage (Rastaldi, Ferrario, Giardino, Dell’Antonio, Grillo, Grillo, Strutz, Muller, Colasanti and D’Amico, 2002).
TGFβ1 is the main inducer of EMT in renal tubular epithelial cells (Fan, et al., 1999, Strutz, Zeisberg, Ziyadeh, Yang, Kalluri, Muller and Neilson, 2002). The expression of FSP1 in transitioning tubular epithelium is induced by TGFβ (Okada, et al., 2000), and tubular basement membrane disintegration leads to TGFβ1 upregulation by mouse proximal tubular epithelial cells contributing to EMT during renal fibrosis (Zeisberg, et al., 2001). Interestingly, TGFβ1-induced EMT in tubular epithelial cells can be reversed by BMP7 by inducing E-cadherin in a SMAD-dependent manner in vitro, and the systemic administration of recombinant human BMP-7 led to repair of damaged renal tubular epithelial cells in a murine model of fibrotic chronic renal injury (Zeisberg, Hanai, Sugimoto, Mammoto, Charytan, Strutz and Kalluri, 2003), indicating that the TGFβ-EMT axis represents a therapeutic target for injury-induced fibrosis.
Pulmonary fibrosis
Lung epithelial cells responding to repeated injury experience persistent inflammation and sustained EMT, leading to fibrosis (Chapman, 2011, Crosby and Waters, 2010). Although the origin of myofibroblasts in lung fibrosis is not certain, some studies have reported the occurrence of EMT in lung fibrosis, partly mediated through TGFβ signaling (Chen, et al., 2015, Kim, et al., 2006, Mubarak, et al., 2012, Willis, et al., 2005, Zhou, et al., 2009, Zolak, et al., 2013). Alveolar epithelial cells (AECs) undergo EMT and contribute to pulmonary fibrosis pathology induced by TGFβ (Kim, Kugler, Wolters, Robillard, Galvez, Brumwell, Sheppard and Chapman, 2006, Willis, Liebler, Luby-Phelps, Nicholson, Crandall, du Bois and Borok, 2005, Zhou, Dada, Wu, Kelly, Trejo, Zhou, Varga and Sznajder, 2009). Moreover, in a TGFβ1 murine model of pulmonary fibrosis, the beta-galactosidase (β-gal)-expressing epithelial cells also expressed mesenchymal markers within injured lungs, indicating epithelial cells as the progenitors for the fibroblasts. Primary AECs cultured on provisional matrix components, fibronectin or fibrin, undergo EMT via integrin-dependent activation of endogenous latent TGFβ1 indicating that the ECM acts as a regulator in the EMT process during fibrogenesis (Kim, Kugler, Wolters, Robillard, Galvez, Brumwell, Sheppard and Chapman, 2006). Exposure of TGFβ to rat primary AECs increased expression of mesenchymal cell markers and a fibroblastic-phenotype, an effect accelerated by TNFα. In vivo, AECs co-expressed epithelial markers and α-smooth muscle actin in lung tissue samples from patients with idiopathic pulmonary fibrosis (IPF) (Willis, Liebler, Luby-Phelps, Nicholson, Crandall, du Bois and Borok, 2005). Studies have also demonstrated that pleural mesothelial cells (PMC) are capable of transitioning into myofibroblasts, a process thought to be driven by TGFβ (Chen, Ye, Zhang, Li, Song, Yang, Mu, Rao, Cai, Xiang, Zhang, Su, Xin and Ma, 2015, Zolak, Jagirdar, Surolia, Karki, Oliva, Hock, Guroji, Ding, Liu, Bolisetty, Agarwal, Thannickal and Antony, 2013). PMC is seen in lung tissue of IPF patients, and labelled PMCs injected into mice traveled to IPF lungs and displayed myofibroblast phenotypic markers in response to TGFβ, the numbers of which correlated with the degree of fibrosis and IPF disease severity (Mubarak, Montes-Worboys, Regev, Nasreen, Mohammed, Faruqi, Hensel, Baz, Akindipe, Fernandez-Bussy, Nathan and Antony, 2012). Increased production of type I collagen, mesenchymal phenotypic markers, and decreased epithelial phenotypic markers are features of PMCs in the bleomycin animal model of injury-triggered pulmonary fibrosis, which is phenotypically similar to human IPF. Moreover, in this model, PMC migration was mediated sboth in vivo and in vitro by TGFβ1-SMAD2/3 signaling (Chen, Ye, Zhang, Li, Song, Yang, Mu, Rao, Cai, Xiang, Zhang, Su, Xin and Ma, 2015).
Cardiac fibrosis
Following cardiac injury, EMT appears to play a role in regeneration or fibrosis to produce mesenchymal cells with both stem cell and myofibroblast characteristics (Limana, et al., 2007). Adult epicardium-derived cells have been shown to reactivate post myocardial injury, undergo EMT and migrate into the injured myocardium where they produce different cell types in vivo, including cardiac interstitial fibroblasts and coronary smooth muscle cells that aid in the cardiac repair process (Limana, Zacheo, Mocini, Mangoni, Borsellino, Diamantini, De Mori, Battistini, Vigna, Santini, Loiaconi, Pompilio, Germani and Capogrossi, 2007, Mikawa and Fischman, 1992, Mikawa and Gourdie, 1996, Poelmann, et al., 1993, Smart, et al., 2013, Winter, et al., 2007). There is also evidence to support the positive regulation of epicardial cell transformation and smooth muscle differentiation by TGFβ, as human adult epicardial cells with an epithelial-like phenotype expressing the cell surface marker vascular cell adhesion marker (VCAM-1) spontaneously underwent EMT and adopted a smooth muscle-like phenotype in vitro activated by TGFβ1 receptor signaling and inhibited by VCAM-1 (Moore, et al., 1999). Furthermore, in epicardium explant studies, both TGFβ1 and TGFβ2 induced loss of epithelial cell markers cytokeratin and membrane-associated Zonula Occludens-1 from epicardial cells, and triggered gain of smooth muscle markers calponin and caldesmon and this was dependent on ALK5 kinase activity, culminating in the induction of epicardial cell EMT and invasion (Compton, et al., 2006).
Hepatic fibrosis
Chronic liver disease gives rise to hepatic fibrosis, but the origin of the activated myofibroblasts is still under debate, and various epithelial cells undergoing EMT may serve as the sources. Hepatic stellate cells (HSCs) are one cellular candidate for activated myofibroblasts (Friedman, et al., 1985), adopting a spindle-shaped phenotype and expressing α-smooth muscle actin and type I collagen (Gressner and Weiskirchen, 2006, Lee, et al., 1995). Lineage tracing experiments in mice have demonstrated that HSCs contribute to 82–96% of myofibroblasts mediating fibrogenesis (Mederacke, et al., 2013). Epithelial hepatocytes and cholangiocytes are also likely candidates for contributing to the myofibroblast population in liver fibrosis. Interestingly, mouse cholangiocytes, co-cultured with myofibroblastic HSCs undergo EMT in vitro, exhibiting increased cell migration, reduced epithelial markers, and induction of mesenchymal markers (Omenetti, Porrello, Jung, Yang, Popov, Choi, Witek, Alpini, Venter, Vandongen, Syn, Baroni, Benedetti, Schuppan and Diehl, 2008).
As in the kidney and lung, TGFβ may be involved in the induction of the EMT phenotype in liver fibrosis. In one study, EMT was induced in hepatocytes in vitro via activation of the TGFβ1/SMAD pathway (Kaimori, et al., 2007). Additional lineage-tracing experiments using transgenic mice demonstrated that TGFβ1 induced hepatocytes to undergo EMT and contributed to the population of FSP1-positive fibroblasts in CCL4-induced model of liver fibrosis, an effect that could be blocked by BMP-7 administration. Also, human cultured intrahepatic epithelial cells treated with TGFβ were shown to undergo EMT-like changes, adopting an invasive fibroblast-type phenotype with loss of cytokeratin-7 and gain of SMAD2/3, S100A4 and α-smooth muscle actin expression. In the same study, TGFβ mRNA and nuclear phospho-SMAD2/3 were highly expressed in damaged ducts of chronic diseased liver tissue that also expressed S100A4, vimentin and MMP-2. Finally, co-expression of epithelial and mesenchymal markers in biliary epithelial cells and cholangiocytes of chronic liver disease patients also supports an in vivo role for TGFβ-induced EMT in human hepatic fibrosis (Diaz, et al., 2008, Rygiel, et al., 2008).
Scleroderma and skin fibrosis
Scleroderma (Sc) is a systemic disorder characterized by autoimmunity, chronic inflammation, vasculopathy and extensive skin and organ fibrosis of unknown etiology (Gazi, et al., 2007). In Sc, early vascular injury precedes fibrosis, and as with renal fibrosis, the persistently activated myofibroblast drives TGFβ-induced gene expression and increases pro-fibrotic cytokine and protease production (Postlethwaite, et al., 2004). Although the origin of the myofibroblasts in Sc fibrotic skin is unknown, studies have once again indicated that the EMT process is one possible source (Postlethwaite, Shigemitsu and Kanangat, 2004). Indeed, increased nuclear translocation of myocardin related transcription factor-A (MRTF-A), a key mechano-responsive transcription factor that signals EMT, has been observed in Sc epidermis (O’Connor and Gomez, 2013, Shiwen, et al., 2015).
Increased levels of TGFβ1, TGFβ receptors, and enhanced TGFβ signaling has been reported in Sc (Dong, et al., 2002, Leask, et al., 2002) thus supporting a role for this cytokine in myofibroblast activation and in the pathogenesis in the fibrosis observed in Sc (Xu, et al., 2009). In one murine model, active TGFβ signaling was enhanced, leading to skin fibrosis that resembled the biochemical, clinical and histologic features of human Sc (Sonnylal, et al., 2007).
In Sc epidermis, keratinocytes have been shown to adopt an activated phenotype associated with active SMAD/TGFβ signaling and display increased expression of pro-fibrotic factors connective tissue growth factor (CTGF) and SNAIL1 expression (Nikitorowicz-Buniak, et al., 2015). Sc keratinocytes stimulated fibroblasts to increase ECM contractility and growth factor expression, the effects of which were dependent on elevated levels of IL-1α expression by epidermal cells and induction of endothelin-1 (ET-1) and TGFβ in fibroblasts (Aden, et al., 2010). In vitro, Sc fibroblasts display enhanced collagen deposition and ECM contraction and remodeling (Jimenez, et al., 1986).
Less is known regarding the contribution of EMT processes to fibrotic skin conditions other than scleroderma. High expression of the mesenchymal marker FSP1 was found in the epidermis and dermis of human hypertrophic scars, which was accompanied by increased levels of inflammatory cytokines, fibrotic markers and EMT-related Slug and TWIST. In this way, a link was demonstrated between unresolved inflammation and the development EMT characteristics during fibrogenesis in hypertrophic scar tissue in vivo (Yan, Grimm, Garner, Qin, Travis, Tan and Han, 2010).
CONCLUSIONS AND FUTURE DIRECTIONS
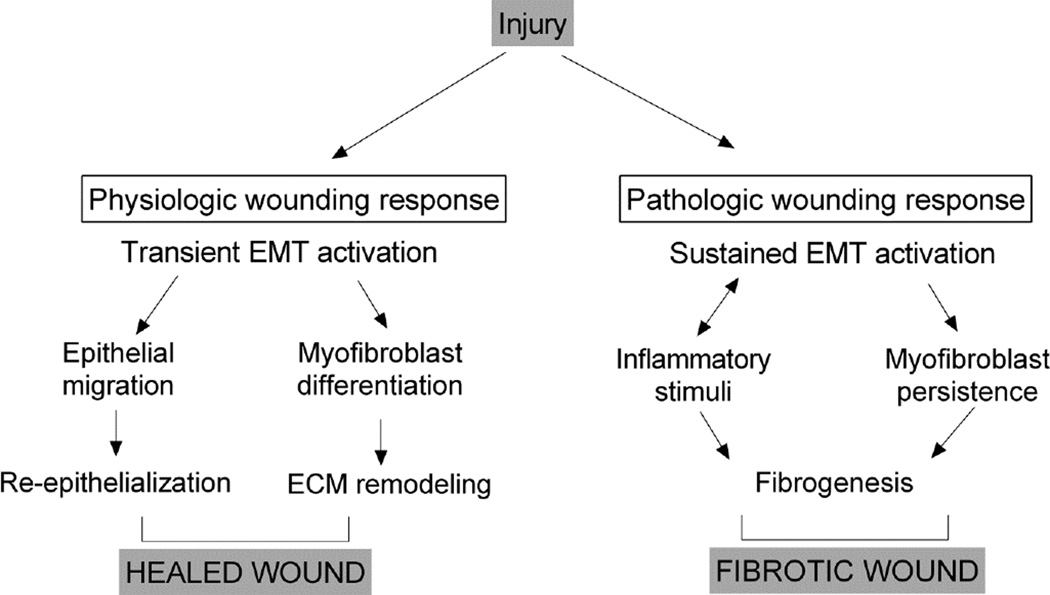
Injury triggers the inflammatory wound healing cascade, and pathologically sustained inflammation is tightly associated with fibrogenesis. This review has summarized evidence that EMT plays a role in physiologic tissue repair, and that sustained EMT is a key mechanism underlying the fibrotic pathology of multiple organs. Given the fundamental parallels between the regulation and signaling of EMT and critical wound-healing processes, it is quite conceivable that early and prolonged activation of EMT in the context of the response to injury promotes inflammation and fibrogenesis that culminates in non-healing wounds of many epithelial tissues (Figure 2). In investigating this hypothesis further, it will be important to keep in mind that EMT is a dynamic and reversible process and cells cannot always be classified as purely epithelial or mesenchymal, especially in vivo, as they may carry features of each. Loss-of epithelial and gain-of-mesenchymal features can also occur simultaneously. Nevertheless, assessing the presence of the classic EMT biomarkers in non-healing tissues and organs in vivo will be critical to define the role for EMT in initiating and sustaining a poor healing response, and may represent a way forward to potential targeting of EMT as a novel and global therapeutic approach for difficult-to-treat wounds.
Fig. 2.
Model for injury-triggered EMT activation in physiologic wound repair (left) and fibrotic wound healing (right).
Acknowledgments
Acknowledgements and Funding: Our research is supported by the National Institutes of Health grants NR015649, NR013881 and DK098055.
REFERENCES
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. Journal of immunology (Baltimore, Md : 1950) 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Aden N, Nuttall A, Shiwen X, de Winter P, Leask A, Black CM, Denton CP, Abraham DJ, Stratton RJ. Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis. The Journal of investigative dermatology. 2010;130:2191–2200. doi: 10.1038/jid.2010.120. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. American journal of physiology Cell physiology. 2006;290:C1532–C1542. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends in cell biology. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- Arnoux V, Come C, Kusewitt D, Hudson L, Savagner P. Cutaneous wound reepithelialization: a partial and reversible EMT. In: Savagner P, editor. Rise and fall of epithelial phenotype: concepts of epithelial-mesenchymal transition. Berlin: Springer; 2005. pp. 111–134. [Google Scholar]
- Arnoux V, Nassour M, L’Helgoualc’h A, Hipskind RA, Savagner P. Erk5 controls Slug expression and keratinocyte activation during wound healing. Mol Biol Cell. 2008;19:4738–4749. doi: 10.1091/mbc.E07-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22:569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. discussion 686. [DOI] [PubMed] [Google Scholar]
- Billottet C, Tuefferd M, Gentien D, Rapinat A, Thiery JP, Broet P, Jouanneau J. Modulation of several waves of gene expression during FGF-1 induced epithelial-mesenchymal transition of carcinoma cells. Journal of cellular biochemistry. 2008;104:826–839. doi: 10.1002/jcb.21667. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. The New England journal of medicine. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Developmental biology. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Carretero M, Escamez MJ, Garcia M, Duarte B, Holguin A, Retamosa L, Jorcano JL, Rio MD, Larcher F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. The Journal of investigative dermatology. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Gutkind JS. Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing. Oral diseases. 2013;19:551–558. doi: 10.1111/odi.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annual review of physiology. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Ye H, Zhang Q, Li FZ, Song LJ, Yang J, Mu Q, Rao SS, Cai PC, Xiang F, Zhang JC, Su Y, Xin JB, Ma WL. Bleomycin induced epithelial-mesenchymal transition (EMT) in pleural mesothelial cells. Toxicology and applied pharmacology. 2015;283:75–82. doi: 10.1016/j.taap.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. The Journal of cell biology. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development (Cambridge, England) 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Wound epithelialization: accelerating the pace of discovery. The Journal of investigative dermatology. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. American journal of physiology Lung cellular and molecular physiology. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. The Journal of cell biology. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. The American journal of pathology. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Di Vita G, Patti R, D’Agostino P, Caruso G, Arcara M, Buscemi S, Bonventre S, Ferlazzo V, Arcoleo F, Cillari E. Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006;14:259–264. doi: 10.1111/j.1743-6109.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- Diaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, Russo PA, Rand EB, Furth EE, Wells RG. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Human pathology. 2008;39:102–115. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem cells (Dayton, Ohio) 2003;21:514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhu S, Wang T, Yoon W, Li Z, Alvarez RJ, ten Dijke P, White B, Wigley FM, Goldschmidt-Clermont PJ. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3908–3913. doi: 10.1073/pnas.062010399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Drake CJ, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Experimental hematology. 2006;34:219–229. doi: 10.1016/j.exphem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science translational medicine. 2014;6:265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney international. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circulation research. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. The Journal of pathology. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gawronska-Kozak B, Grabowska A, Kur-Piotrowska A, Kopcewicz M. Foxn1 Transcription Factor Regulates Wound Healing of Skin through Promoting Epithelial-Mesenchymal Transition. PloS one. 2016;11:e0150635. doi: 10.1371/journal.pone.0150635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi H, Pope JE, Clements P, Medsger TA, Martin RW, Merkel PA, Kahaleh B, Wollheim FA, Baron M, Csuka ME, Emery P, Belch JF, Hayat S, Lally EV, Korn JH, Czirjak L, Herrick A, Voskuyl AE, Bruehlmann P, Inanc M, Furst DE, Black C, Ellman MH, Moreland LW, Rothfield NF, Hsu V, Mayes M, McKown KM, Krieg T, Siebold JR. Outcome measurements in scleroderma: results from a delphi exercise. The Journal of rheumatology. 2007;34:501–509. [PubMed] [Google Scholar]
- Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. Journal of cell science. 1999;112(Pt 24):4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- Golinko MS, Joffe R, de Vinck D, Chandrasekaran E, Stojadinovic O, Barrientos S, Vukelic S, Tomic-Canic M, Brem H. Surgical pathology to describe the clinical margin of debridement of chronic wounds using a wound electronic medical record. Journal of the American College of Surgeons. 2009;209:254–260. doi: 10.1016/j.jamcollsurg.2009.04.012. e251. [DOI] [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. Journal of cellular and molecular medicine. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. The EMBO journal. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama R, Nakao S, Shibusawa Y, Ishikawa O, Moro T, Mikami K, Fukumitsu H, Ueda Y, Minakawa K, Tabata Y, Bou-Gharios G, Inagaki Y. Differential contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis in mice. The Journal of investigative dermatology. 2011;131:529–536. doi: 10.1038/jid.2010.314. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. The Journal of clinical investigation. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thrombosis and haemostasis. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. The Journal of biological chemistry. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. Journal of cell science. 2012;125:4417–4422. doi: 10.1242/jcs.099697. [DOI] [PubMed] [Google Scholar]
- Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2) Journal of dermatological science. 2009;56:19–26. doi: 10.1016/j.jdermsci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American journal of pathology. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Okada H, Takenaka T, Watanabe Y, Suzuki H. A case report suggesting the occurrence of epithelial-mesenchymal transition in obstructive nephropathy. Clinical and experimental nephrology. 2009;13:385–388. doi: 10.1007/s10157-009-0168-4. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. The Journal of clinical investigation. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H, Grunert S. Autocrine PDGFR signaling promotes mammary cancer metastasis. The Journal of clinical investigation. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SA, Feldman G, Bashey RI, Bienkowski R, Rosenbloom J. Co-ordinate increase in the expression of type I and type III collagen genes in progressive systemic sclerosis fibroblasts. The Biochemical journal. 1986;237:837–843. doi: 10.1042/bj2370837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell cycle (Georgetown, Tex) 2011;10:2865–2873. doi: 10.4161/cc.10.17.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. The Journal of biological chemistry. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. The Journal of clinical investigation. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Molecular and cellular biology. 2007;27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Yeomanson D, Bryant HE. PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. Journal of pediatric hematology/oncology. 2015;37:245–251. doi: 10.1097/MPH.0000000000000329. [DOI] [PubMed] [Google Scholar]
- Krainock M, Toubat O, Danopoulos S, Beckham A, Warburton D, Kim R. Epicardial Epithelial-to-Mesenchymal Transition in Heart Development and Disease. Journal of clinical medicine. 2016;5 doi: 10.3390/jcm5020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusewitt DF, Choi C, Newkirk KM, Leroy P, Li Y, Chavez MG, Hudson LG. Slug/Snai2 is a downstream mediator of epidermal growth factor receptor-stimulated reepithelialization. The Journal of investigative dermatology. 2009;129:491–495. doi: 10.1038/jid.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. Journal of cell science. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. The Journal of cell biology. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ, Finlay DR, Holmes A, Pennington D, Shi-Wen X, Chen Y, Venstrom K, Dou X, Ponticos M, Black C, Bernabeu C, Jackman JK, Findell PR, Connolly MK. Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis and rheumatism. 2002;46:1857–1865. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. The Journal of cell biology. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. The Journal of clinical investigation. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circulation research. 2007;101:1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nature reviews Nephrology. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer research. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- Lyons JG, Birkedal-Hansen B, Pierson MC, Whitelock JM, Birkedal-Hansen H. Interleukin-1 beta and transforming growth factor-alpha/epidermal growth factor induce expression of M(r) 95,000 type IV collagenase/gelatinase and interstitial fibroblast-type collagenase by rat mucosal keratinocytes. The Journal of biological chemistry. 1993;268:19143–19151. [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science (New York, NY) 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- Mathay C, Giltaire S, Minner F, Bera E, Herin M, Poumay Y. Heparin-binding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds. The Journal of investigative dermatology. 2008;128:717–727. doi: 10.1038/sj.jid.5701069. [DOI] [PubMed] [Google Scholar]
- McCarthy DW, Downing MT, Brigstock DR, Luquette MH, Brown KD, Abad MS, Besner GE. Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. The Journal of investigative dermatology. 1996;106:49–56. doi: 10.1111/1523-1747.ep12327214. [DOI] [PubMed] [Google Scholar]
- McDonald TM, Pascual AS, Uppalapati CK, Cooper KE, Leyva KJ, Hull EE. Zebrafish keratocyte explant cultures as a wound healing model system: differential gene expression & morphological changes support epithelial-mesenchymal transition. Experimental cell research. 2013;319:1815–1827. doi: 10.1016/j.yexcr.2013.03.036. [DOI] [PubMed] [Google Scholar]
- Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nature communications. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental biology. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development (Cambridge, England) 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- Mubarak KK, Montes-Worboys A, Regev D, Nasreen N, Mohammed KA, Faruqi I, Hensel E, Baz MA, Akindipe OA, Fernandez-Bussy S, Nathan SD, Antony VB. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. The European respiratory journal. 2012;39:133–140. doi: 10.1183/09031936.00141010. [DOI] [PubMed] [Google Scholar]
- Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene. 2005;24:4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. TGFbeta3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. The Journal of cell biology. 2003;163:1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitorowicz-Buniak J, Denton CP, Abraham D, Stratton R. Partially Evoked Epithelial-Mesenchymal Transition (EMT) Is Associated with Increased TGFbeta Signaling within Lesional Scleroderma Skin. PloS one. 2015;10:e0134092. doi: 10.1371/journal.pone.0134092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani Y, Iwano M, Yamaguchi Y, Harada K, Nakatani K, Akai Y, Nishino T, Shiiki H, Kanauchi M, Saito Y, Neilson EG. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney international. 2005;68:1078–1085. doi: 10.1111/j.1523-1755.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- O’Connor JW, Gomez EW. Cell adhesion and shape regulate TGF-beta1-induced epithelial-myofibroblast transition via MRTF-A signaling. PloS one. 2013;8:e83188. doi: 10.1371/journal.pone.0083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG. Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney international. 2000;58:587–597. doi: 10.1046/j.1523-1755.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. The American journal of physiology. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. The Journal of clinical investigation. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circulation research. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Current opinion in rheumatology. 2004;16:733–738. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-related cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. The Journal of cell biology. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? Journal of cellular biochemistry. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez H, Patel SB, Pastar I. The Role of TGFbeta Signaling in Wound Epithelialization. Advances in wound care. 2014;3:482–491. doi: 10.1089/wound.2013.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. The American journal of pathology. 2012;180:2188–2200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastaldi MP, Ferrario F, Giardino L, Dell’Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D’Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney international. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Laboratory investigation; a journal of technical methods and pathology. 1993;68:696–707. [PubMed] [Google Scholar]
- Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Laboratory investigation; a journal of technical methods and pathology. 2008;88:112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Experimental cell research. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Sasaki T. The effects of basic fibroblast growth factor and doxorubicin on cultured human skin fibroblasts: relevance to wound healing. The Journal of dermatology. 1992;19:664–666. doi: 10.1111/j.1346-8138.1992.tb03755.x. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. Journal of cellular physiology. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. The Journal of cell biology. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Experimental cell research. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Shirley SH, Hudson LG, He J, Kusewitt DF. The skinny on Slug. Mol Carcinog. 2010;49:851–861. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwen X, Stratton R, Nikitorowicz-Buniak J, Ahmed-Abdi B, Ponticos M, Denton C, Abraham D, Takahashi A, Suki B, Layne MD, Lafyatis R, Smith BD. A Role of Myocardin Related Transcription Factor-A (MRTF-A) in Scleroderma Related Fibrosis. PloS one. 2015;10:e0126015. doi: 10.1371/journal.pone.0126015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Dube KN, Riley PR. Epicardial progenitor cells in cardiac regeneration and neovascularisation. Vascular pharmacology. 2013;58:164–173. doi: 10.1016/j.vph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Smith BN, Bhowmick NA. Role of EMT in Metastasis and Therapy Resistance. Journal of clinical medicine. 2016;5 doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogabe Y, Abe M, Yokoyama Y, Ishikawa O. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006;14:457–462. doi: 10.1111/j.1743-6109.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, Vanpelt CS, Geng YJ, Deng JM, Behringer RR, de Crombrugghe B. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis and rheumatism. 2007;56:334–344. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- Stoll S, Garner W, Elder J. Heparin-binding ligands mediate autocrine epidermal growth factor receptor activation In skin organ culture. The Journal of clinical investigation. 1997;100:1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll SW, Rittie L, Johnson JL, Elder JT. Heparin-binding EGF-like growth factor promotes epithelial-mesenchymal transition in human keratinocytes. The Journal of investigative dermatology. 2012;132:2148–2157. doi: 10.1038/jid.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. Journal of cellular physiology. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney international. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes & development. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nature reviews Molecular cell biology. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tan B, Pascual A, de Beus A, Cooper K, Hull E. TGFbeta (transforming growth factor beta) and keratocyte motility in 24 h zebrafish explant cultures. Cell biology international. 2011;35:1131–1139. doi: 10.1042/CBI20110063. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Terao M, Ishikawa A, Nakahara S, Kimura A, Kato A, Moriwaki K, Kamada Y, Murota H, Taniguchi N, Katayama I, Miyoshi E. Enhanced epithelial-mesenchymal transition-like phenotype in N-acetylglucosaminyltransferase V transgenic mouse skin promotes wound healing. The Journal of biological chemistry. 2011;286:28303–28311. doi: 10.1074/jbc.M111.220376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature reviews Molecular cell biology. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Takayama H, Horiguchi N, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett. 2001;509:95–100. doi: 10.1016/s0014-5793(01)03126-x. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes & development. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles AM, Boyer B, Tarone G, Thiery JP. Alpha 2 beta 1 integrin is required for the collagen and FGF-1 induced cell dispersion in a rat bladder carcinoma cell line. Cell adhesion and communication. 1996;4:187–199. doi: 10.3109/15419069609014222. [DOI] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nature cell biology. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk SW, Iqbal SA, Bayat A. Interactions of the Extracellular Matrix and Progenitor Cells in Cutaneous Wound Healing. Advances in wound care. 2013;2:261–272. doi: 10.1089/wound.2012.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Weil BR, Herrmann JL, Abarbanell AM, Tan J, Markel TA, Kelly ML, Meldrum DR. MEK, p38, and PI-3K mediate cross talk between EGFR and TNFR in enhancing hepatocyte growth factor production from human mesenchymal stem cells. American journal of physiology Cell physiology. 2009;297:C1284–C1293. doi: 10.1152/ajpcell.00183.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. Journal of cell science. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Liu CJ, Fearon ER, Margolis B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene. 2008;27:3875–3879. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. The American journal of pathology. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter EM, Grauss RW, Hogers B, van Tuyn J, van der Geest R, Lie-Venema H, Steijn RV, Maas S, DeRuiter MC, deVries AA, Steendijk P, Doevendans PA, van der Laarse A, Poelmann RE, Schalij MJ, Atsma DE, Gittenberger-de Groot AC. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007;116:917–927. doi: 10.1161/CIRCULATIONAHA.106.668178. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature medicine. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell research. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chen YG, Massague J. The nuclear import function of Smad2 is masked by SARA and unmasked by TGFbeta-dependent phosphorylation. Nature cell biology. 2000;2:559–562. doi: 10.1038/35019649. [DOI] [PubMed] [Google Scholar]
- Yan C, Grimm WA, Garner WL, Qin L, Travis T, Tan N, Han YP. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. The American journal of pathology. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Pursell B, Lu S, Chang TK, Mercurio AM. Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. Journal of cell science. 2009;122:2473–2480. doi: 10.1242/jcs.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Yamaguchi Y, Itami S, Yoshikawa K, Tabata Y, Matsumoto K, Nakamura T. Neutralization of hepatocyte growth factor leads to retarded cutaneous wound healing associated with decreased neovascularization and granulation tissue formation. The Journal of investigative dermatology. 2003;120:335–343. doi: 10.1046/j.1523-1747.2003.12039.x. [DOI] [PubMed] [Google Scholar]
- You S, Avidan O, Tariq A, Ahluwalia I, Stark PC, Kublin CL, Zoukhri D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Invest Ophthalmol Vis Sci. 2012;53:126–135. doi: 10.1167/iovs.11-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. The American journal of pathology. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature medicine. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Zhang ZQ, Liu YY, Zhou X, Shi XH, Jiang Q, Fan DL, Cao C. Requirement of Galphai1/3-Gab1 signaling complex for keratinocyte growth factor-induced PI3K–AKT-mTORC1 activation. The Journal of investigative dermatology. 2015;135:181–191. doi: 10.1038/jid.2014.326. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nature cell biology. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. American journal of physiology Lung cellular and molecular physiology. 2009;297:L1120–L1130. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolak JS, Jagirdar R, Surolia R, Karki S, Oliva O, Hock T, Guroji P, Ding Q, Liu RM, Bolisetty S, Agarwal A, Thannickal VJ, Antony VB. Pleural mesothelial cell differentiation and invasion in fibrogenic lung injury. The American journal of pathology. 2013;182:1239–1247. doi: 10.1016/j.ajpath.2012.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]