FIGURE 1.
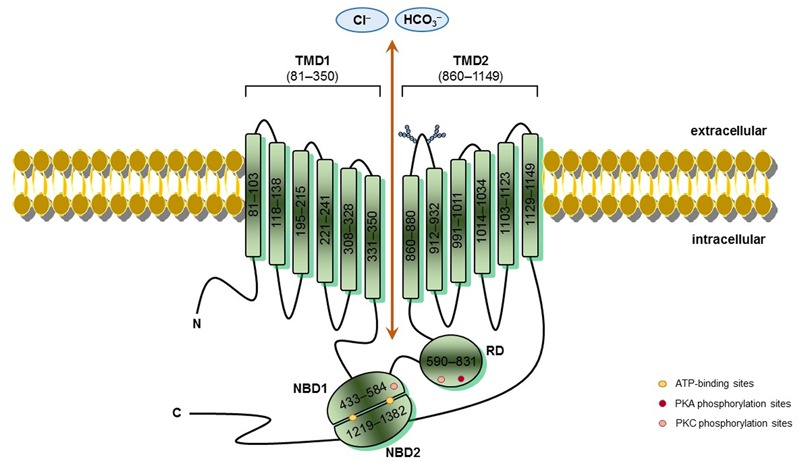
CFTR schematic structure – Cystic fibrosis transmembrane conductance regulator (CFTR) is a 1,480-amino acids protein inserted into the cell surface. CFTR possesses five domains: two transmembrane domains (TMD1/2), containing six hydrophobic alpha-helices, which cross the cell surface lipid bilayer, and are joined by two intracellular loops and three extracellular loops, and with glycosylated residues linked in the extracellular loop 4 (N894, N900); two nucleotide-binding domains (NBD1/2) with highly conserved sequenced for ATP-binding, where occur hydrolysis; and one regulatory domain (RD) with multiple phosphorylation sites. CFTR channel open when protein kinase A (PKA) and protein kinase C (PKC) phosphorylate RD and ATPs bind to side chain charged amino acids in NBDs, thereby activating CFTR function. TMDs form the gate where occurs chloride conductance. The positions denoted into the boxes correspond to the first and last amino acid of each fragment and CFTR sequence was obtained in the Cystic Fibrosis Mutation Database (CFTR1 database; http://www.genet.sickkids.on.ca/Home.html).
