FIGURE 7.
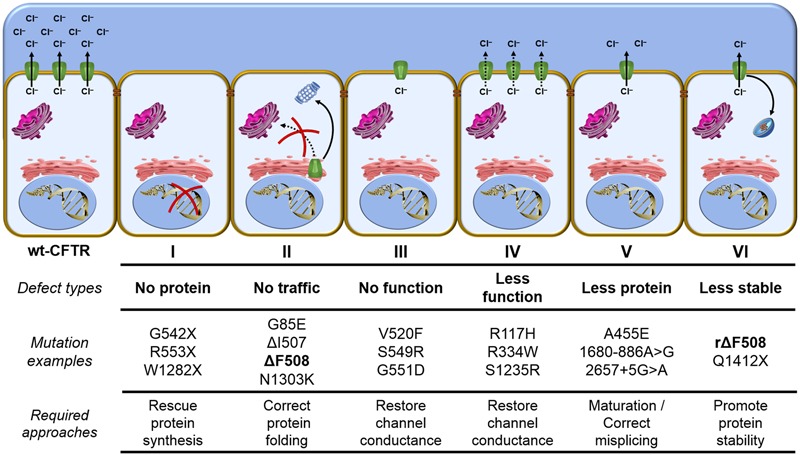
Classes of CFTR mutations – Distribution of CFTR mutations into six functional classes according to the primary molecular defect: Class I mutants are no protein synthesis, since the presence of premature stop codons (class Ia) or frameshifts for deletions or insertions (class Ib) preclude translation of full-length CFTR. Class II mutants are impaired trafficking protein, since CFTR fails to acquire complete folding and ER-associated degradation (ERAD) machinery eliminate the protein. Class III mutants are defective channel gating, since CFTR reach the cell surface, but it does not exhibit channel gating due to diminished ATP binding and hydrolysis. Class IV mutants are less functional proteins, since channel amount that achieve the plasma membrane could be similar to wt-CFTR, but it presents reduced chloride conductance. Class V mutants are less protein maturation caused by amino acid substitution or alternative splicing, since the protein amount that reaches the cell surface is reduced and it also leads to loss of chloride transport due to reduction in the quantity of CFTR channels. Class VI mutants are less stable protein, since CFTR at the plasma membrane is removed during the recycling and it is sent for lysosome degradation. wt, wild type; CFTR, cystic fibrosis transmembrane conductance regulator; rΔF508, rescued ΔF508 by low-temperature incubation; and ER, endoplasmic reticulum.
