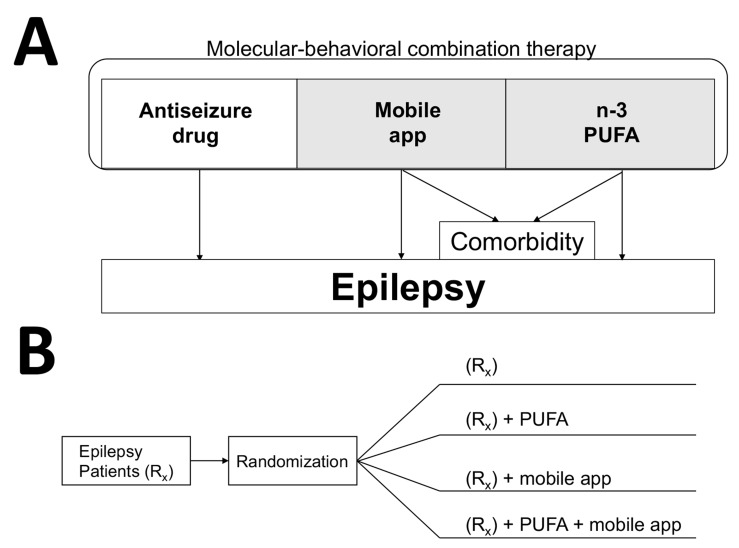
Fig. (4).
Molecular-behavioral combination therapy for epilepsy patients. (A) Three components of the molecular-behavioral combination therapy are antiseizure pharmaceutical drug, n-3 PUFA as pharmacologically-active incentives, and mobile app delivering antiseizure music and epilepsy self-management content [3]. Given high prevalence of depression as comorbidity for chronic disorders, the add-on therapy (in shaded area) can be specifically prescribed for patients with refractory epilepsy and experiencing symptoms of depression. Mobile apps and software for treating depression and anxiety show promise in clinical trials [211-213]. In addition to n-3 PUFA, curcumin also can be developed as psychobehavioral incentives due to its antiseizure and antidepressant activities. (B) An example of a parallel study design to test the molecular-behavioral combination therapy in form of a drug-device combination product. Patients with refractory epilepsy are usually given two or more antiseizure drugs (Rx group). To establish efficacy of all possible combinations, such four-arm study can last 6-12 months, and can further include crossover, as was previously used to establish efficacy of n-3 PUFA in patients with refractory epilepsy [60], or/and a delayed start/prerandomization to reduce placebo responses [214]. Determining pharmacokinetic interactions between natural products and antiseizure drugs are important to ensure the safety of the drug-drug combination study (2nd and 4th arm), even though natural products may have prescription medicine status (e.g. n-3 PUFA as Lovaza® or Vascepa®).