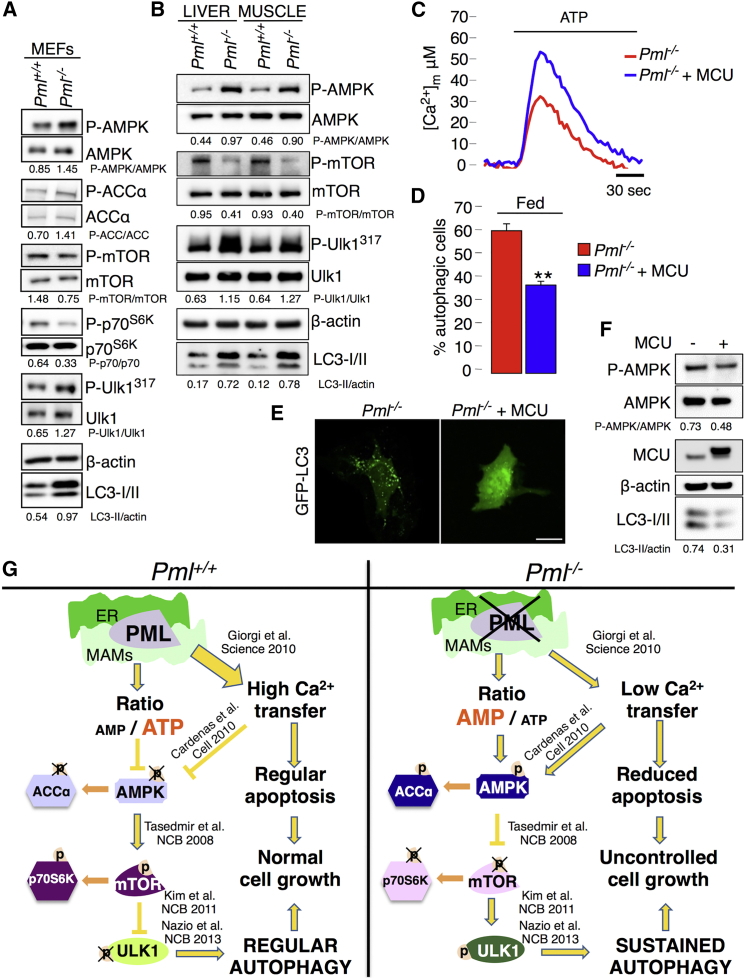
Figure 4.
PML Modulates Autophagy through the AMPK/mTOR/Ulk1 Pathway in a Ca2+-Dependent Manner
(A) Immunoblot detection of the phosphorylation status of AMPK, ACCα, p70S6K, mTOR, and Ulk1 in Pml+/+ and Pml−/− MEFs.
(B) Detection of autophagy and AMPK-mTOR-Ulk1 phosphorylation levels in the liver and skeletal muscle of Pml+/+ and Pml−/− mice.
(C) Representative traces of increased mitochondrial Ca2+ levels in Pml−/− MEFs after MCU overexpression. Pml−/−: [Ca2+]m peak 33.7 ± 2.55; Pml−/− + MCU: [Ca2+]m peak 59.2 ± 6.22) SEM. ∗∗p < 0.01, n = 3.
(D–F) Quantification of autophagy in Pml−/− MEFs following MCU overexpression via (D and E) analysis of GFP-LC3 puncta or (F) immunoblotting. Representative images are shown. Bars, SEM. ∗∗p < 0.01, n = 3. Scale bar, 10 μm.
(G) Schematic model of autophagy regulation by PML. In the absence of Pml, the release of Ca2+ from the ER into the mitochondria and the production of ATP are reduced. This low-energy status induces AMPK activation, mTOR inhibition, and Ulk1 phosphorylation, leading to increased autophagy.