Figure 5.
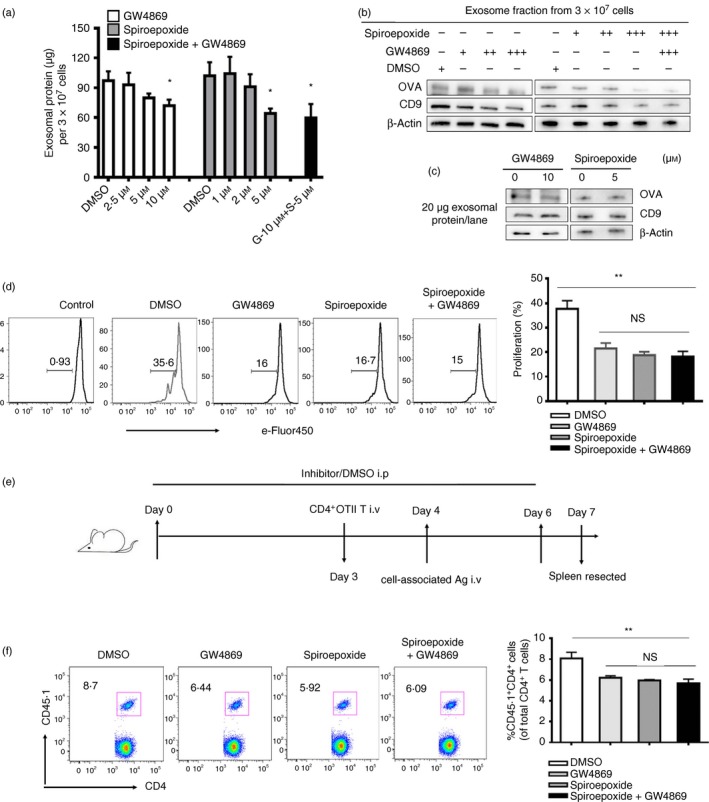
Inhibition of exosome secretion decreased the proliferation of antigen‐specific CD4+ T cells both in vitro and in vivo. (a) RAW264.7 cells were incubated with control (DMSO) or with 2·5 μm (+), 5 μm (++), 10 μm (+++) GW4869 or/and 1 μm (+), 2 μm (++), 5 μm (+++) spiroepoxide, and purified exosomes from supernatants of equal cell numbers of control‐ or inhibitor‐treated macrophages (Mφs) were quantified by a BCA assay. (b) Purified exosomes secreted by equal number of DMSO‐treated or inhibitor‐treated RAW264.7 cells were analysed by immunoblotting for the presence of exosomal marker CD9 and ovalbumin (OVA). (c) Equal amounts of exosomal proteins (quantified by BCA assay) secreted by either control‐ or inhibitor‐treated RAW264.7 cells cultured with OVA were analysed by immunoblotting for the presence of CD9 and OVA protein. (d) eFluor‐450 dye‐labelled naive CD4+ OT‐II T cells, naive dendritic cells (DCs) from naive mice and Mφs from OVA/cell‐injected mice were co‐cultured in the presence of DMSO (solvent), or GW4869 (10 μm) or/and spiroepoxide (5 μm) for 3 days. The proliferation of OT‐II T cells was analysed by flow cytometry. (e) CD45.2 mice were intraperitoneally injected with GW4869 or/and spiroepoxide (1·25 mg/kg) or DMSO once a day for 6 days, and the mice were intravenously administered 2 × 106 CD45.1+ CD4+ Vβ5+ Vα2+ T cells on the fourth day followed by injection of OVA/cells after 1 day. Three days after the OVA/cells injection, proliferation of CD45.1+ CD4+ T cells was analysed by FACS (f). Data are shown as the mean ± SD of three to five mice per group and representative of three independent experiments. Two‐tailed Student's t‐test was used for data analysis. *P < 0·05, **P < 0·01.
