Table 2.
Pathogenetic differences between ALS-FUS and FTLD-FUS
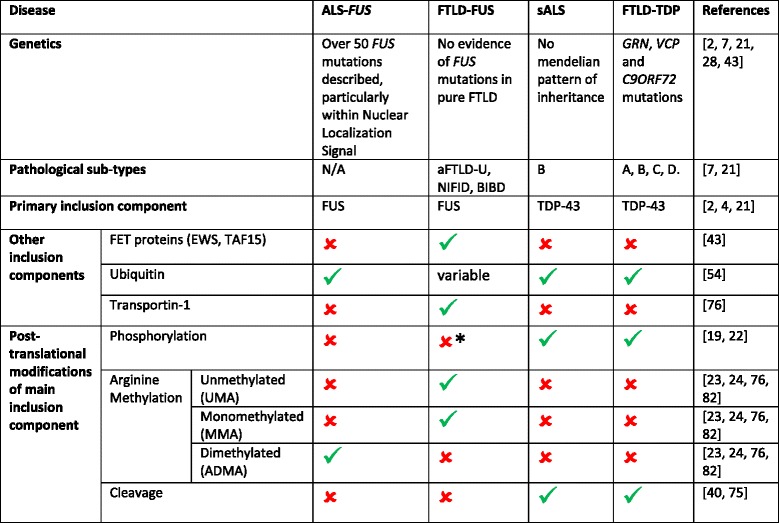
Under normal physiological conditions, FUS does not appear to undergo any post-translational modifications. Arginine methylation is facilitated by protein methyltransferase 1 (PRMT1), which inhibits binding to Transportin-1 and prevents nuclear re-localization. Hypermethylation of FUS protein only occurs in ALS-FUS, and not FTLD-FUS, suggesting possible insight into the way FUS aggregates are the predominant pathological characteristic of FTLD-FUS despite the absence of causative mutations, * = FUS is phosphorylated in response to DNA damage, some evidence of DNA damage in FTLD
