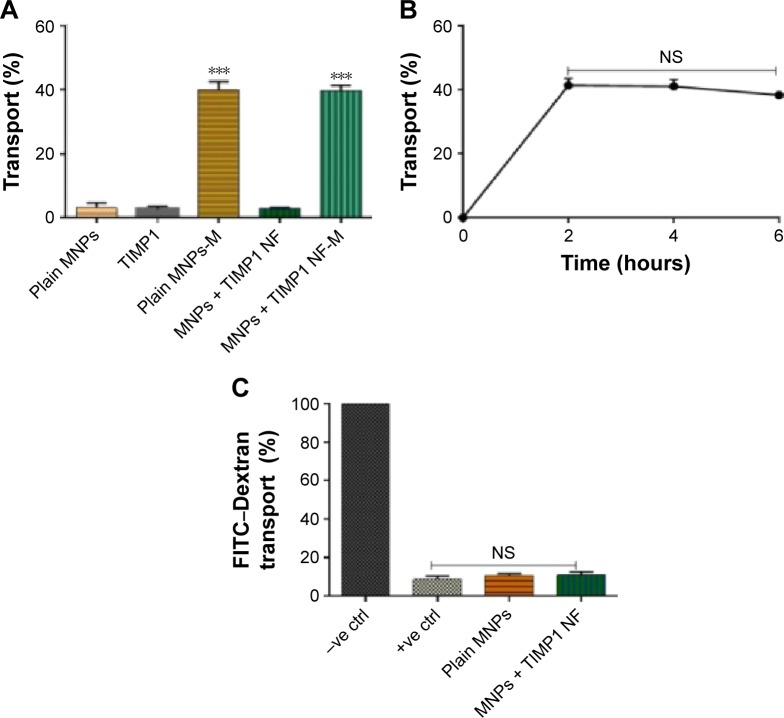
Figure 2.
MNPs + TIMP1 nanoformulation (NF) transmigration study using in vitro BBB model.
Notes: (A) Transmigration efficiency evaluation: MNPs, free TIMP1, and MNPs + TIMP1 NF were added in the upper chamber of the transwells, and samples were tested for transmigration ability in the presence or absence of static magnet (0.8 T) placed underneath the cell culture plate for the 2 hours treatment; transmigrated samples were collected from the bottom chamber, centrifuged, and estimated for iron content using iron assay; (B) effect of magnetic treatment time with respect to NF transmigration efficiency: samples from the lower chamber at different time points, ie, 2, 4, and 6 hours, were collected, and transmigrated MNPs + TIMP1 NF was collected from the bottom chamber, centrifuged, and calculated for iron content using iron assay. (C) BBB integrity analysis using the FITC–dextran transport assay: FITC–dextran transport was measured for MNPs/MNPs + TIMP1 NF after 2 hours’ magnetic treatment, and FITC–dextran (molecular weight 40,000 Da) was added to the upper chamber of the insert. After 30 minutes’ incubation, relative fluorescence unit from the lower chamber of the transwell was measured. Results were expressed as % FITC–dextran transport with respect to the untreated control cultures (−ve control = no cell and +ve control = with all BBB cells) and represented as mean ± SE of independent experiments. ***P≤0.001.
Abbreviations: BBB, blood–brain barrier; SE, standard error; FITC, fluorescein isothiocyanate; TIMP1, tissue inhibitor of metalloproteinase-1; NF, nanoformulation; MNPs, magnetic nanoparticles; NS, not significant; ctrl, control; M, Magnet.