Abstract
Mice are routinely used to study the development of the external genitalia and, in particular, the process of male urethral closure. This is because misplacement of the male penile urethra, or hypospadias, is amongst the most common birth defects reported in humans. While mice present a tractable model to study penile development, several structures differ between mice and humans, and there is a lack of consensus in the literature on their annotation and developmental origins. Defining the ontology of the mouse prepuce is especially important for the relevance and interpretation of mouse models of hypospadias to human conditions. We have developed a detailed annotation of the adult mouse penis that addresses these differences and enables an accurate comparison of murine and human hypospadias phenotypes. Through MRI data, gross morphology and section histology, we define the origin of the mouse external and internal prepuces, their relationship to the single human foreskin as well as provide a comprehensive view of the various structures of the mouse penis and their associated muscle attachments within the body. These data are combined to annotate structures in a novel 3D adult penis atlas that can be downloaded, viewed at any angle, and manipulated to examine the relationship of various structures.
Keywords: 3D model, Mouse, MRI, Penis, Penis atlas, Prepuce
Introduction
Hypospadias, the ectopic placement of the urethral meatus along the ventral aspect of the penis, is one of the most common birth defects in live male births [Nassar et al., 2007]. Despite its prevalence, there is still relatively little known about the factors that mediate urethral closure. In order to better define the etiology of this condition, hypospadias has been modeled in the mouse using knockout and transgenic lines as well as hormonal manipulations. However, interpretation of the resulting phenotypes is confounded by the overall differences in morphology between the mouse and human penis. Describing the anatomy of the mouse penis is essential for defining the relevance of murine studies to human penis and urethral development as well as for the classification of hypospadias phenotypes. Excellent progress has already been made in defining the anatomy of the mouse penis. Detailed descriptions of structures not seen in humans, such as the male urogenital mating protuberance (MUMP; a cartilaginous element that extends distally from the mouse penis) [Yang et al., 2010; Rodriguez et al., 2011] and external prepuce (a fur-covered skin flap that protects the retracted penis) [Blaschko et al., 2013] have particularly contributed to our understanding of mouse penis morphology. However, there still remains confusion and inaccuracies regarding the annotation and embryological origin of basic adult mouse penis structures.
In mice, as in humans, the penis develops as a result of androgen-driven masculinization of the genital tubercle (GT) [Yamada et al., 2003, 2006; Schlomer et al., 2013]. However, the adult mouse penis is completely internalized in its flaccid state as compared to the externalized human penis [Blaschko et al., 2013]. Humans have a single prepuce structure that covers the glans when the penis is in the flaccid state. Conversely, mice, like many eutherian mammals, appear to have 2 separate prepuce structures. Recent studies have adopted the use of the nomenclature external prepuce to refer to the hair-covered external covering of the penis, and internal prepuce to refer to the structure that retracts over the glans [Blaschko et al., 2013]. However, there is still sparse information regarding the embryological origin of both these structures as well as their analogy to the single prepuce structure seen in humans. Adding further confusion, the internal prepuce is often misidentified, or confused with the greatly elongated glans seen in mice [Blaschko et al., 2013; Hutson et al., 2014], while other studies fail to identify the structure, referring to just external prepuce [Rodriguez et al., 2011; Schlomer et al., 2013].
The single prepuce in humans is almost always affected by hypospadias, even in the most common and mild, anterior forms of the disease where the urethra opens just beneath its usual location or at the base of the glans [Blaschko et al., 2012]. Similarly, prepuce defects are seen both in moderate (with the urethral opening along the penile shaft) and severe hypospadias forms (where the urethra opens at the base of the penile shaft, between the scrotal bulges, or at the base of the scrotum) [Baskin, 2004]. In all cases, the human foreskin forms as a dorsal hood over the glans, due to its deficient development and fusion on the ventral aspect of the penis. The dorsal hood foreskin is often the first indication of hypospadias, especially in the more mild forms of the disease [Leung and Robson, 2007]. Given the importance of this structure in the diagnosis and pathology of human hypospadias, it is essential to understand how this relates to the 2 prepuce structures seen in mice. Classifying mouse hypospadias phenotypes is further complicated by the lack of consensus in the literature as to what constitutes the glans, the shaft and the internal prepuce, making an accurate distinction between mild (glandular), intermediate (shaft), and severe (penoscrotal) hypospadias difficult [Baskin, 2000; Seifert et al., 2008]. Furthermore, the lack of consensus in annotation and descriptions makes comparisons of hypospadias phenotypes between different mouse models nearly impossible.
The aim of this study was to define the ontology of the internal and external prepuce in mice and provide a comprehensive anatomical reference of the adult mouse penis, paying particular attention to the glans, os penis and MUMP, which differ between mice and humans. We then place these structures in the context of the internalized and externalized mouse penis. We have performed high-resolution MRI scans of the flaccid penis in situ and the externalized penis to create a 3D penis atlas that can be examined in any plane to further facilitate the identification and placement of these structures for researchers in the field. This is complemented with section histology to clearly annotate and describe the anatomy of the mouse penis, particularly focusing on structures relevant to its use as a model for human hypospadias.
Materials and Methods
Tissue Collection
Wild-type mice (FVB/NJ) were bred in the animal facility in the Department of Zoology at the University of Melbourne. Mice were handled and killed according to the guidelines established by the National Health and Medical Research Council of Australia (2004) and protocols were approved by The University of Melbourne Animal Experimentation and Ethics Committees.
An adult penis was collected from a mouse at 43 days postnatal (P43), without the external prepuce, and fixed overnight in 4% paraformaldehyde for ex vivo MRI. The tissue was washed in PBS 2× for 30 min each and stored in PBS containing 4 mm of gadopentetate dimeglumine (Gd, Magnevist), an MRI contrast agent that allows faster imaging, before being embedded in a 10-ml Falcon tube in 2% agarose containing 1 mm of Gd. One adult P43 mouse was also used for MRI of the penis in situ scanning immediately postmortem. Low-resolution scans of 2 additional adult (P43) wild-type mice were also performed to ensure no unique variations were present in the penis used to render the 3D image.
Penises used for section immunohistochemistry were collected from P43 (n = 4) and P47 (n = 2) adult male mice and fixed overnight in either 4% paraformaldehyde or Bouin’s fixative. The tissue was then stored in 0.5 m ethylenediaminetetraacetic acid for 4 days to decalcify the os penis and ameliorate tearing of the sample during sectioning. The decalcified tissues were then dehydrated with ethanol before processing in paraffin wax.
Gross morphology pictures of adult mouse penises (P43 and P47) were taken immediately postmortem by applying gentle pressure to the body wall on either side of the external prepuce, causing the penis to extend, revealing the structures normally covered by the external prepuce in the flaccid state.
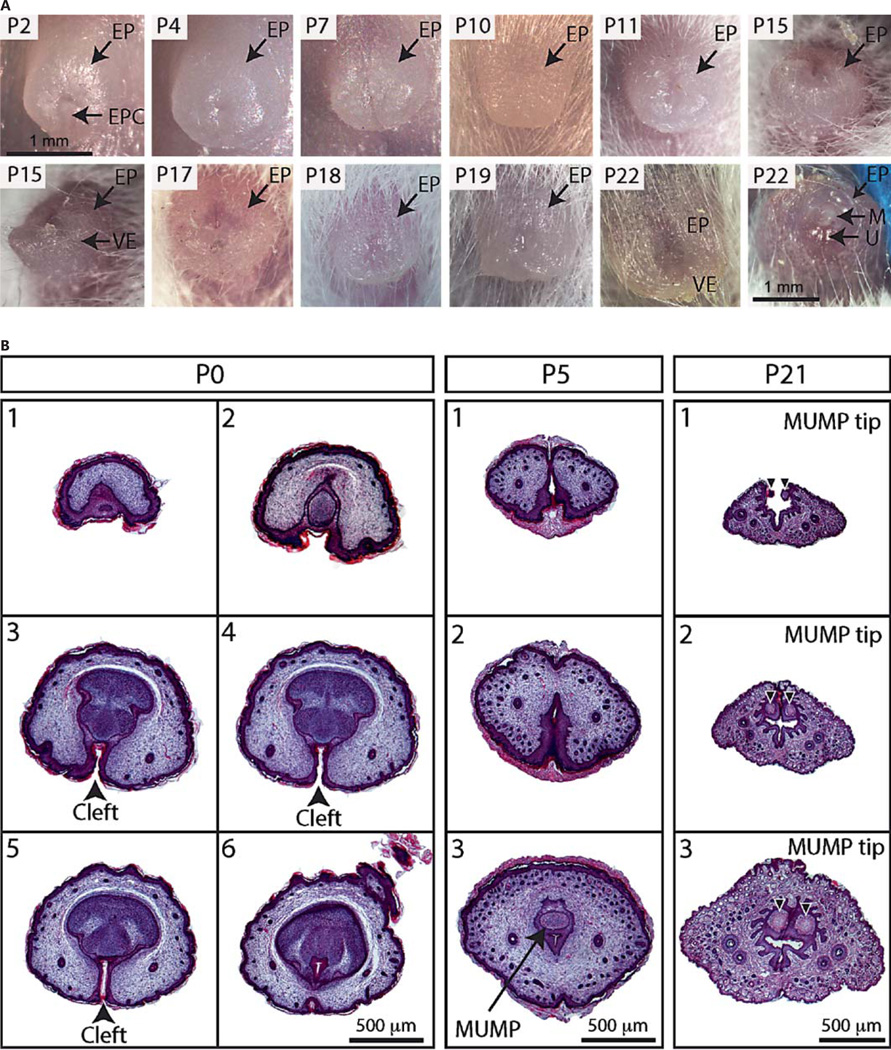
The development of the external and internal prepuce was examined by gross morphology and section histology of GTs collected from mice starting at P0 and continuing until P24 (P0–P22 shown in fig. 2). We examined n = 3 penises histologically at the chosen time points of P0, P5, P7, P10, P21, and P24, and n = 1 was checked for consistency at the intervening time points of P2, P4, P8, P9, P11, P14–P19, P22, and P23. Together, these time points span the majority of the differentiation of the mouse penis. We did not observe any overt differences between wild-type individuals. Gross morphology pictures were taken of the intact GTs before the tissue was fixed overnight in 4% paraformaldehyde, dehydrated with ethanol and processed in paraffin wax for section histology.
Fig. 2.
A Gross morphology of the GT from P2–P22 showing the development of the EP. B Hematoxylin- and eosin-stained transverse sections of the distal most aspects of the developing GT at representative stages. The EP is initially unfused, resulting in a ventral prepuce cleft (black arrowheads in P0 sections). The first sign of cartilage of the os penis and future MUMP can be seen at P5 along with dense hair follicle development (arrow). By P21, the most distal and bifid aspect of the MUMP (black arrowheads) and the G can be seen protruding through the EP. EPC = External prepuce cleft; M = MUMP; VE = ventral extension; U = urethral meatus.
The hypospadias mutant was isolated from a transgene insertional mutagenesis screen [Xie et al., 2007]. The condition is heritable, autosomal and homozygous recessive. The urethra is open along the entire glans, resulting in a urethral opening at the body wall. Gross morphology pictures were taken of the penis in its native state.
Section Histology
Paraffin-embedded penises were serially sectioned at 7 µm and stained with hematoxylin and eosin or Mallory’s trichrome according to standard methods. Images were obtained using a Nikon DS-5M-L1 Digital Sight Camera System.
MRI Acquisition
Both in situ and ex vivo imaging were performed with a 4.7-T Bruker Avance III scanner with 30 cm horizontal bore (Bruker BioSpin GmbH, Ettlingen, Germany) and BGA12S2 gradient set (Bruker).
In situ MRI
An adult P43 mouse was positioned on a purpose-built animal holder in the prone position with an actively decoupled 4-channel surface receive coil (Bruker) positioned over the penis for in situ imaging. The imaging protocol consisted of a 3-plane localizer sequence followed by multislice axial, coronal and sagittal images to accurately determine the orientation and position of the mouse penis within the magnet.
The final image volume was acquired with a rapid acquisition with relaxation enhancement (RARE) sequence and the following imaging parameters: repetition time (TR) = 2,000 ms, RARE factor = 8, effective echo time (TEeff) = 60 ms, field of view (FOV) = 19.2 × 19.2 × 9.6 mm, matrix size = 256 × 256 × 64, and spatial resolution = 75 × 75 × 150 µm.
Ex vivo MRI
A cryogenically cooled, 2-channel, transmit/receive surface coil (half shell, diameter 20 mm, Bruker) was positioned directly over an adult P43 mouse penis for ex vivo imaging. The same procedures as for in situ imaging were used to determine the position of the penis within the magnet. The final high-resolution 3D image was acquired using a fast low-angle shot sequence with the following imaging parameters: TR = 200 ms, TE = 9.5 ms, FOV = 6.4 × 6.4 × 2.56 mm, matrix size = 320 × 320 ×128, isotropic spatial resolution = 20 × 20 × 20 µm, and number of repetitions = 18. Repetitions were averaged offline using MATLAB (2012b, Math-Works, Natick, Mass., USA) to improve the signal to noise ratio.
MRI Analyses
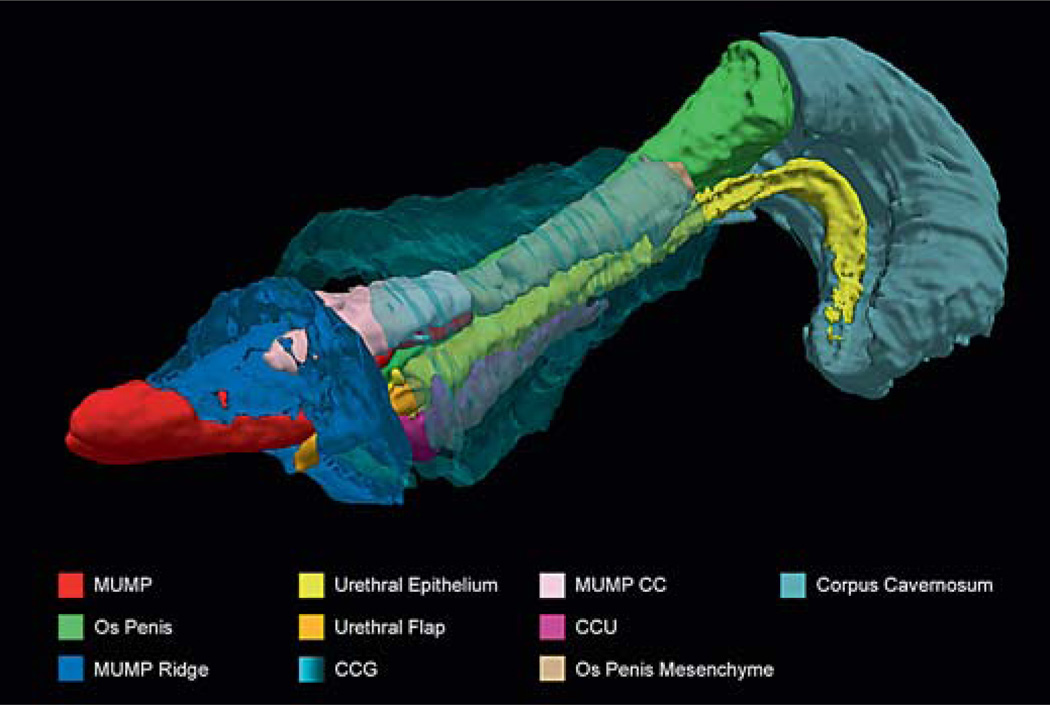
Regions of interest (ROIs) were outlined on the ex vivo image using ITK-SNAP [Yushkevich et al., 2006] and included the MUMP, MUMP ridge, os penis, corpus cavernosum (CC), corpus cavernosum glandis (CCG), corpus cavernosum urethrae (CCU), urethral epithelium, urethral flaps, and os penis mesenchyme. ROIs were interpreted conservatively, so areas that were ambiguous, such as the definitive border between the MUMP ridge and the glans, and the proximal division between urethral epithelium and CC, were omitted. A 3D render of these ROIs was generated using Paraview (http://www.paraview.org/).
Results
Defining the Mouse Internal and External Prepuce
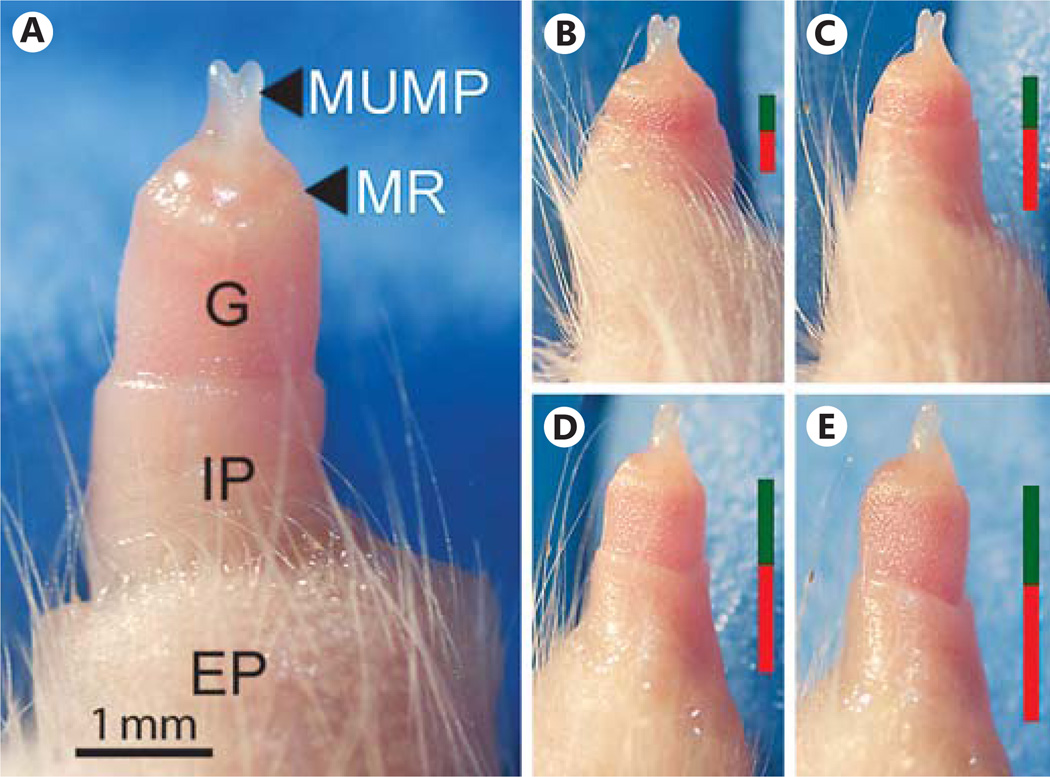
The adult mouse penis has 2 prepuce structures that cover the glans and penile body (fig. 1A). During extension, the penis protrudes through the external prepuce. As the penis elongates, there is a protraction of the internal prepuce down the glans and along the penile body (fig. 1B–E). In the relaxed, internalized state, the penis sits within the retracted internal prepuce (now adjacent to the glans) and the external prepuce (which is now positioned distal to the phallus).
Fig. 1.
A Gross morphology of an adult (P47) mouse penis externalized by applying gentle pressure on the abdomen. The glans (G) is extended through the external prepuce (EP) and away from the body wall as the internal prepuce (IP) protracts down the glans. Also indicated is the MUMP ridge (MR) – the boundary between the distal aspect of the G, through which the MUMP projects, and proximal G. B–E illustrates the protraction of the IP as the penis extends away from the body wall when pressure is applied. Bars on the right of B–E indicate the relative contribution of IP (red) and G (green) to penis length.
Developmental Origin and Ontology of the Internal and External Prepuce
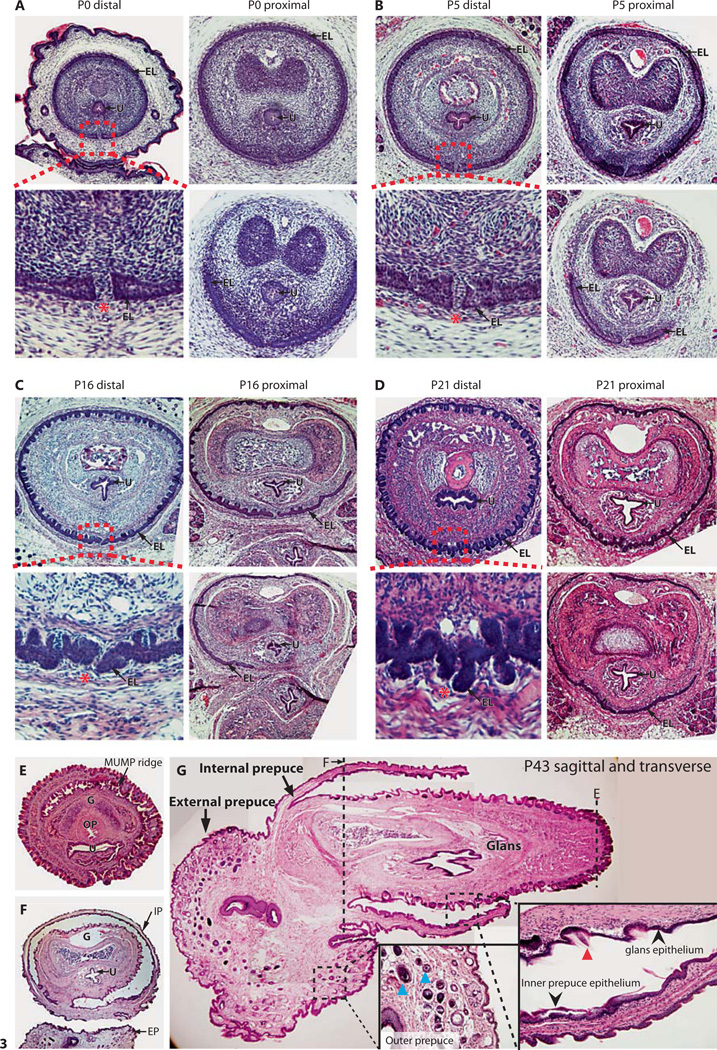
In order to define the developmental origins of the mouse prepuces, we observed the development of the internal and external prepuce using gross morphological examination of the developing GT from the day of birth to P22 (fig. 2A) and section histology (fig. 2B, 3A–D). The gross morphology shows that the external prepuce in mice is derived from the neonatal prepuce of the GT, which is formed from the growth of the preputial swelling to cover the glans. During the first 2 weeks of postnatal development, the external prepuce grows and elongates, especially on the ventral aspect (fig. 2A). Hair first becomes evident around P10, but the hair follicles are already forming at birth (fig. 2B). Histology of the most distal tip of the developing GT (fig. 2B) shows that the external prepuce is open on the ventral aspect and is only fused proximally at birth. As the penis elongates, the external prepuce fuses more distally, fully enclosing the glans, with only the tip of the MUMP reaching the opening in the relaxed state at P22 (fig. 2A). The cleft on the distal tip of the ventral aspect of the external prepuce remains into adulthood and is the opening through which the penis extends in the erect state (fig. 1).
Fig. 3.
A–D Hematoxylin- and eosin-stained transverse sections of the internalized mouse penis as it develops postnatally. Red-dotted boxes indicate the terminus of the internal preputial epithelial lamina (EL), and show the site of fusion of the G and EP. Red asterisks indicate the site of preputial attachment. E, F Transverse hematoxylin- and eosin-stained sections of an adult (P47) penis in the externalized state. E Distal section showing the G, MUMP ridge, urethra (U), and os penis (OP), whereas F is more proximal and shows the IP surrounding the G and the EP. G Sagittal section of an adult (P47) mouse penis partially externalized. Red arrowhead indicates penile spines of the G, black arrowheads show the smooth mucosal epithelium of the IP, and blue arrowheads show hair follicles of the EP. Dashed lines indicate corresponding plane of transverse sections shown in E and F. For further abbreviations, see figures 1 and 2.
Histology of the GT shows the independent formation of the internal prepuce adjacent to the glans. It originates from the internal preputial epithelial lamina, which delaminates from the glans to form the internal prepuce. The internal preputial epithelial lamina is present at birth as a discontinuous structure surrounding the developing glans, except at the most ventral aspect of the penis where the glans remains fused to the external prepuce. The ventral fusion of the glans to the external prepuce extends along the entire length of the GT (fig. 3A–C) and persists up until around P21 (fig. 3D). The ventral fusion of the internal preputial epithelial lamina after P21 (fig. 3D,E) detaches the glans from the external prepuce and allows the phallus to extend (fig. 2A, P22). Similarly in adulthood, the phallus can be readily extended and the glans shows no attachment to the external prepuce. The internal preputial epithelial lamina delaminates to form the glans epithelium, characterized by epithelial spines, and the internal prepuce epithelium which has a classical mucosa structure. The external prepuce has a typical skin epithelial structure with dense hair follicles (fig. 3E).
Detailed Analysis of the Gross Morphology of the Adult Mouse Penis
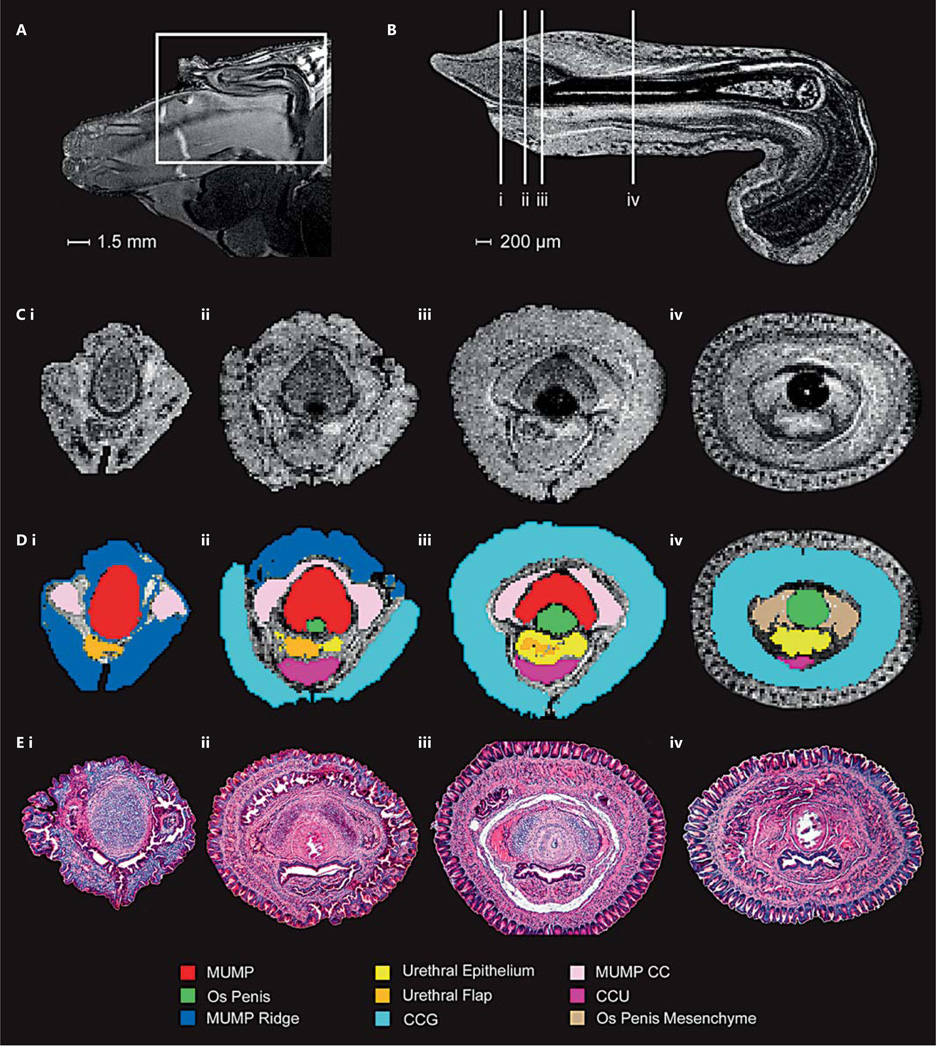
MRI was performed on an adult mouse and a fixed, externalized penis to determine the relationship of different tissue types of the penis as well as its native position within the body (fig. 4A,B). Examining the externalized penis MRI data in 3 planes [dorsal-ventral, left-right (sagittal), proximal-distal (transverse)] enabled the demarcation of the various structures. Proximal-distal MRI slices and their corresponding shaded ROIs are shown in conjunction with relative histological sections (fig. 4C–E). The compiled ROIs create a 3D penis atlas (fig. 5; online supplementary atlas; for all supplementary material, see www.karger.com/doi/10.1159/000431010, http://hdl.handle.net/11343/54723) showing the exact relative positioning of different tissues in scale. The high isotropic resolution of the ex vivo MRI allows the adult mouse penis to be viewed from any angle, while the in situ scan shows its natural internalized state within the body (online suppl. videos). This enables the viewing of the histological structures from multiple angles. In addition, the fully proportional 3D rendering highlights the different structures, which can be viewed from any angle, either individually or in any chosen combination.
Fig. 4.
A Whole-mount MRI slice of the adult mouse penis (P43) in situ and B a mid-sagittal plane MRI slice of the externalized penis. The white box in A shows the location of the penis beneath the EP and extending into the body. The 90° bend in the penis is associated with its attachment to muscles that aid in erection. The vertical white lines (B) indicate the plane of transverse sections shown in C and E. C Still frame images from the transverse plane of the MRI of the externalized penis from distal to proximal (i–iv). D Transverse MRI frames from C with shaded regions of interest, color-coded as indicated in the diagram key. E Corresponding transverse hematoxylin- and eosin-stained stained sections from a P43 externalized mouse penis. For abbreviations, see figures 1 and 2.
Fig. 5.
3D penis reconstruction using ROIs highlighted in the original MRI (fig. 4). This penis was externalized upon dissection, so the IP is protracted (not shown) and the EP has been removed. Note, the CCU and MUMP ridge extend further proximally than shown (as can be seen in section histology). The gradients between the proximal most aspects of these structures and the surrounding tissue were too ambiguous to interpret and, thus, are not fully annotated in the color code of the MRI. MUMP CC = MUMP corpora cavernosa.
The Flaccid Penis in situ
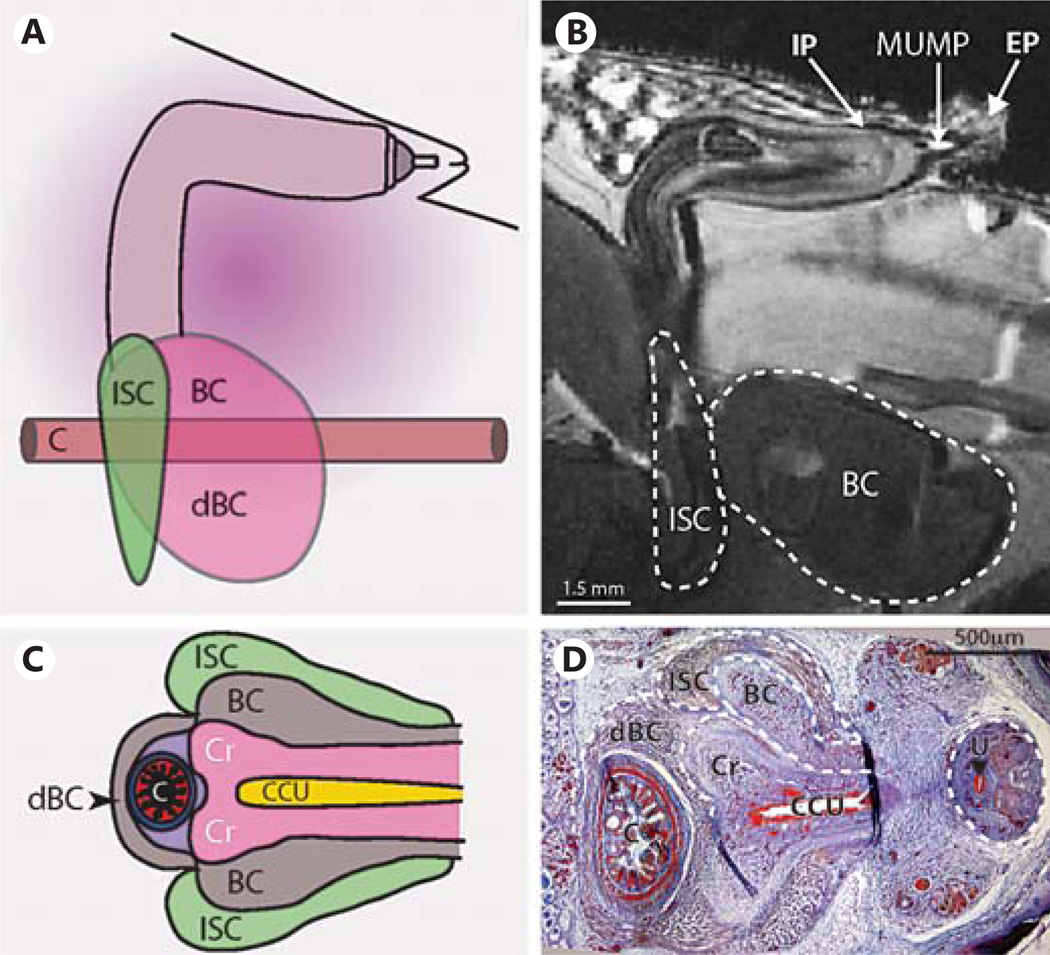
Using in situ MRI and section histology, we further examined the internalized penis to annotate its position relative to the body wall and its associated muscle attachments. In the native flaccid state, the MUMP extends slightly beyond the body wall and into the external prepuce. The 90° bend in the penis can clearly be seen (fig. 6B and shown diagrammatically in fig. 6A), after which the CC of the penile body widens and terminates into the diverging crura, which attaches to the pubic bone via the ischiocavernosus muscle (fig. 6). The enlarged terminal penile body, the urethral bulb, is the site of attachment for the bulbocavernosus muscle (BC), which extends laterally to encircle the colon and extends to the rectum (fig. 6C,D).
Fig. 6.
A Diagram showing the adult mouse penis from a sagittal view as it sits in the abdomen in the flaccid state. This view is also seen in B as an MRI still frame from the whole-mount penis in situ (P43) scans. Dashed lines outline the BC and ischiocavernosus muscle. The location of the IP, EP and MUMP are indicated. C Diagram of the transverse view of the terminal penis and its muscle attachments. This view is seen also in D Mallory’s trichrome staining of a P0 transverse section. Dashed lines outline the various muscles and terminating penile body, while the dashed line circle on the right outlines proximal penile G. C = Colon; Cr = crura, the terminus of the penile body; dBC = dorsal bulbocavernosus muscle; ISC = ischiocavernosus muscle; U = urethra (black arrowhead). For further abbreviations, see figures 1 and 2.
Defining Hypospadias Phenotypes
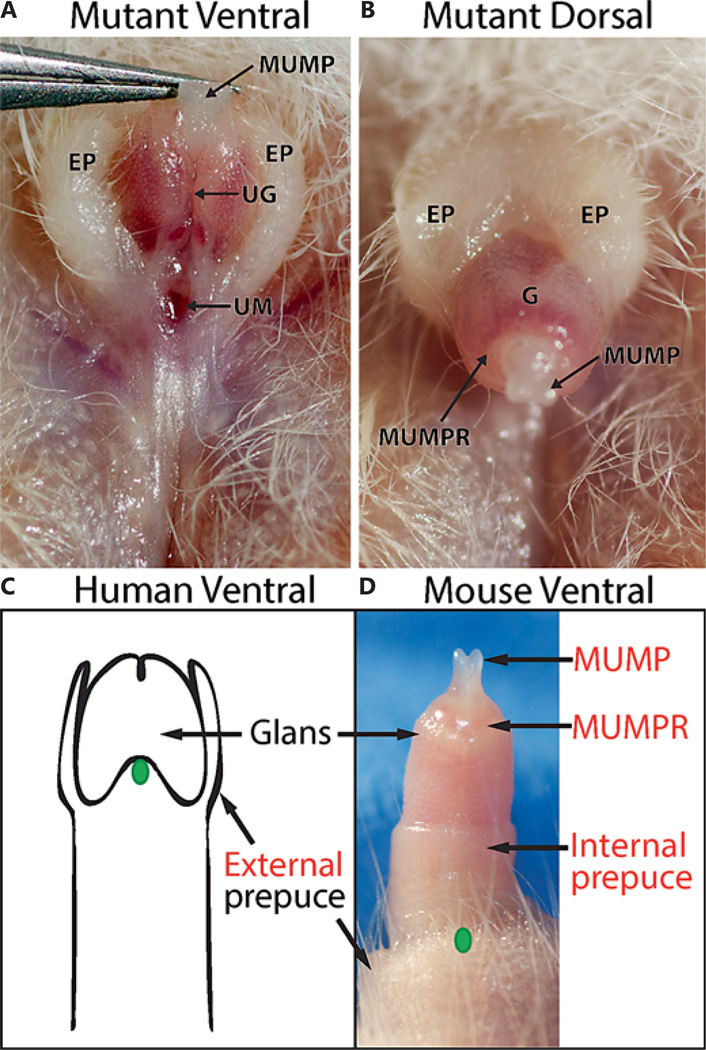
An example of a hypospadias phenotype is shown in figure 7. The adult presents with a dorsal external foreskin hood, which leaves the glans of the penis partially exposed even in the relaxed state (fig. 7A). Lifting the penis up with forceps reveals a misplaced urethral opening at the base of the glans (fig. 7B). Based on the human clinical scale, this would be analogous to a mild, subcoronal hypospadias.
Fig. 7.
Hypospadias in the adult mouse penis. The ventral aspect (A) is viewed with forceps holding the MUMP to expose the urethral meatus at the base of the G. The EP fails to fuse at the ventral aspect of the penis, creating the dorsal hood seen in A and B. C Diagram of the adult human penis with the foreskin cut away to show the G beneath. The position of the UM in a similar subcoronal hypospadias phenotype to that shown in A and B is indicated by the green oval. D The normal mouse penis, partially extended, with the location of the urethral opening relative to the normal histology is indicated by the green oval. Labels in black indicate shared and analogous structures between the mouse and the human penis, while those in red are mouse specific. MUMPR = MUMP ridge; UG = urethral groove; UM = urethral meatus. For further abbreviations, see figures 1 and 2.
Discussion
Through the use of MRI and 3D rendering, we provide the first comprehensive atlas of the adult mouse penis. These data collectively present a novel way to view the various structures of the adult mouse penis while maintaining the proportions between structures as well as their relation to the penis and whole body. We have utilized this technology, along with histology and gross morphology, to generate a comprehensive description of the adult mouse penis that not only reflects the most recent research on this organ, but also contributes new descriptions of the structure and embryological origin of the external and internal prepuces, critical for the utility of this model for human hypospadias.
Gross Morphology of the Adult Mouse Penis
The anatomy of the adult mouse penis will be described, starting with the most distal aspect and continuing proximally until it terminates in the abdomen. In keeping with current anatomical descriptions, the adult mouse penis is comprised of a penile body, glans, MUMP, and the internal and external prepuces (fig. 1). The cartilaginous MUMP protrudes beyond the tip of the glans and is partially bifid at the distal end. The MUMP is a distal extension of the os penis, an ossified bone, and is flanked proximally by spongy tissue called the MUMP CC, separate from the spongy tissue found in the body of the glans (fig. 4C–E, 5) [Blaschko et al., 2013]. The MUMP ridge and associated MUMP ridge groove have been previously characterized [Rodriguez et al., 2011] as an invagination that encircles the tip of the glans, at the base of the MUMP projection. The MUMP ridge groove divides the distal glans from the MUMP ridge and can be clearly seen in distal section histology of the adult penis (fig. 3E) as well as in the 3D rendering of the penis from the MRI (fig. 5). The MUMP ridge groove has been mistaken for the internal preputial space [Blaschko et al., 2013] which is a much more distally located structure (fig. 1, 3G). The MUMP ridge groove instead represents a fissure at the tip of the glans, while the MUMP ridge is a continuation of the glans demarcated only superficially by the MUMP ridge groove (fig. 3G).
The urethral meatus in mice terminates as a ventral cleft that is located in the dorsal MUMP ridge (fig. 4C–E). At P25 and older, 2 projections, termed the urethral flaps, can be seen inside the urethral opening and extend out from the urethral epithelium at the distal tip of the male urethra (fig. 4B, E ii, 5) [Rodriguez et al., 2011].
The glans of the penis extends from the bend of the penile body distally to the MUMP ridge [Rodriguez et al., 2011] and is covered in keratinized epithelial projections called penile spines [Murakami, 1987]. The glans contains the CCG, urethra, associated CCU, and the skeletal elements of the os penis [Murakami, 1987]. The CCG is erectile tissue that fuses medially into a continuous ring encircling the os penis giving structure and rigidity to the erect penis (fig. 4C–E iv). The CCG does not extend past the glandular ridge distally, and it tapers off proximally in the glans as the os penis enlarges (fig. 4B, 5).
The glandular urethra is directly bordered by the os penis dorsally and the CCU ventrally. At its most distal end, the urethra extends beyond the glans to the meatus in the MUMP ridge region. Proximally, the urethra continues from the glans into the penile body. The CCU terminates at the distal tip of the glans (fig. 4B) and follows the urethra proximally through the length of the penis and into the urethral bulb (fig. 6). The CCU is thought to be analogous to the corpus spongiosum in the human penis [Rodriguez et al., 2011].
Connected to the os penis bone of the glans is the CC that extends into the penile body (fig. 4B, 5). The penile body also contains the proximal aspect of the urethra and CCU. The penile body continues into the abdomen with a posterior right-angle bend, down towards the pelvis [Murakami, 1987] (fig. 4A, 6). When the penis is in its relaxed state, it is positioned beneath the hair-bearing external prepuce in a cavity lined by the smooth mucosa of the internal prepuce. As the glans of the penis extends away from the body wall (and through the external prepuce), the internal foreskin protracts midway along the glans on the elongating penile shaft (fig. 1B–E).
Postnatal Development of the External and Internal Prepuces
The outer layer of the GT at birth is formed from the preputial swellings that grow to cover the glans of the developing GT. It is this structure that forms the external prepuce in mice. The internal prepuce forms later, within the GT as the glans separates from the preputial lamina. The complete circularization of the internal preputial epithelial lamina occurs between 22 and 24 days after birth (fig. 3D shows P21; P24 not shown), and precedes the delamination of the internal prepuce from the glans [Blaschko et al., 2013]. Only after circularization of the internal prepuce and its subsequent delamination from the glans, can the glans penis extend through the external prepuce.
The external prepuce shows a marked extension of the ventral aspect of the tissue, evident from day 10 postpartum and shows sparse hair coverage at birth. The external prepuce tissue in the adult phallus is composed of normal hair-bearing skin epithelial tissue. In contrast, the internal prepuce is a typical mucosal epithelium. Although they are different in composition, the internal prepuce is a continuation of the external prepuce, not unlike the epithelial transition seen in the inner and outer foreskin of humans [McCoombe and Short, 2006]. In mice, the connection between these 2 structures is at the beginning of the internal prepuce tissue in proximal sections of the GT and in the internalized penis (fig. 3A–E).
Comparisons of Human and Mouse Prepuce
The internal prepuce in mice is analogous in function to the single human prepuce, as they both cover and protect the glans. Likewise, during induced extension (and presumably natural erection) in mice, the internal foreskin protracts over the glans of the penis, akin to the human foreskin (fig. 1, 3E). However, the human foreskin forms from the preputial swellings on the outer surface of the GT, while the inner foreskin mucosa in mice is derived from a delamination of the glans from the prepuce inside the GT. Based on developmental ontology, the outer foreskin of mice represents the analogous structure to the human prepuce. This ontology is in line with observations of hypospadias in mice (such as the example shown in fig. 7A,B) that result in the hooded external prepuce morphology typically seen in the foreskin of human hypospadias cases.
The timing of prepuce maturation relative to birth is also different in mice and humans. Human penises typically have a fully formed and ventrally fused foreskin epithelium at birth, whereas in mice the internal foreskin does not fully detach until after P21 (fig. 3). The external prepuce is fused proximally in mice at birth, but fusion at the distal tip appears to continue as the penis matures and becomes fully virilized (fig. 2B).
Proximal Attachments to the Penis within the Abdomen
The CC of the penile body terminates into the diverging crura, which attach to the pubic bone via the ischiocavernosus muscle [Yiou et al., 2001] (fig. 6A). In between the crura is the urethral bulb, the enlarged terminal region of the penile body that contains the CCU and urethra. The 2 lateral masses of the BC insert into the urethral bulb and cover the entire surface of the pelvic outlet. The BC extends laterally into 2 projections that encircle the colon and distally the rectum (fig. 6). These projections have been previously described as the levator ani muscle due to their position in surrounding the rectum as in humans. However, since their role in mice is not in lifting the anus [Holmes and Sachs, 1994; Poortmans and Wyndaele, 1998; Yiou et al., 2001], we have adopted the functionally more accurate term of the dorsal bulbocavernosus muscle. Together, the mouse BC and dorsal bulbocavernosus muscles facilitate erection by increasing pressure of the penile bulb [Elmore and Sachs, 1988; Holmes and Sachs, 1994].
Redefining Hypospadias Phenotypes
Having examined the origin and function of the internal and external prepuce in mice, it is possible to redefine mouse hypospadias phenotypes in relation to human clinical classifications. What appears to be a very severe hypospadias phenotype in mice can, in fact, be exaggerated by the greatly extended glans and lack of external penile shaft. Thus, a mild subcoronal hypospadias with the urethral meatus located at the base of the glans in mice will present as a meatus located in close proximity to the body with a large patent urethral groove in the glans. This appears as a more dramatically pronounced phenotype to that seen in subcoronal forms in humans. A biological example of this is shown in figure 7. The urethral meatus is located at the base of the glans (fig. 7A,B) consistent with a mild form of human hypospadias, despite its close proximity to the body. In humans, the penile shaft sits outside the body (fig. 7C), while in mice, the penile body is largely internalized. Thus, it is unclear if an intermediate hypospadias form analogous to a shaft urethral meatus in humans can be seen in mice. Finally, severe hypospadias phenotypes have been observed in mice with the urethral opening in the scrotal region resulting in a bifid scrotum [Dravis et al., 2004; Seifert et al., 2009]. The lack of intermediate or mid-shaft hypospadias mutants in mice does not present a major barrier to the utility of mouse models, since such phenotypes are quite rare.
Summary
We present a novel anatomical reconstruction of the adult mouse penis with careful annotation and ontology of the internal and external prepuce. These data provide an important framework for the continued use of mouse models of hypospadias and, in particular, their interpretation to human clinical manifestations. In addition, we provide the first context for the normal positioning of flaccid penis within the abdomen. Our high-resolution MRI data provide a novel resource for the urology community, enabling virtual dissection of the mouse penis in 3 planes as well as visualization of individual structures in a 3D–rotating model. We also place the muscles involved in erection into the context of the flaccid mouse phallus.
Supplementary Material
Acknowledgments
The authors acknowledge the facilities and the scientific and technical assistance of the National Imaging Facility at the Florey Institute of Neuroscience and Mental Health, Parkville. Research reported in this publication was supported by The National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK096263.
References
- Baskin LS. Hypospadias and urethral development. J Urol. 2000;163:951–956. [PubMed] [Google Scholar]
- Baskin LS. Hypospadias and Genital Development. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 3–5. [Google Scholar]
- Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation. 2012;84:261–268. doi: 10.1016/j.diff.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, et al. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestroltreated mice. Anat Rec (Hoboken) 2013;296:1127–1141. doi: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Elmore LA, Sachs BD. Role of the bulbospongiosus muscles in sexual behavior and fertility in the house mouse. Physiol Behav. 1988;44:125–129. doi: 10.1016/0031-9384(88)90355-1. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Sachs BD. Physiology and mechanics of rat levator ani muscle: evidence for a sexual function. Physiol Behav. 1994;55:255–266. doi: 10.1016/0031-9384(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Baskin LS, Risbridger G, Cunha GR. The power and perils of animal models with urogenital anomalies: handle with care. J Pediatr Urol. 2014;10:699–705. doi: 10.1016/j.jpurol.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKC, Robson WLM. Hypospadias: an update. Asian J Androl. 2007;9:16–22. doi: 10.1111/j.1745-7262.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat. 1987;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Nassar N, Bower C, Barker A. Increasing prevalence of hypospadias in Western Australia, 1980–2000. Arch Dis Child. 2007;92:580–584. doi: 10.1136/adc.2006.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poortmans A, Wyndaele JJ. M. levator ani in the rat: does it really lift the anus? Anat Rec. 1998;251:20–27. doi: 10.1002/(SICI)1097-0185(199805)251:1<20::AID-AR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Jr, Weiss DA, Yang JH, Menshenina J, Ferretti M, et al. New insights on the morphology of adult mouse penis. Biol Reprod. 2011;85:1216–1221. doi: 10.1095/biolreprod.111.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomer BJ, Feretti M, Rodriguez E, Jr, Blaschko S, Cunha G, Baskin L. Sexual differentiation in the male and female mouse from days 0 to 21: a detailed and novel morphometric description. J Urol. 2013;190(Suppl 4):1610–1617. doi: 10.1016/j.juro.2013.02.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009;136:3949–3957. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Chen H, Overbeek PA, Reneker LW. Elevated insulin signaling disrupts the growth and differentiation pattern of the mouse lens. Mol Vis. 2007;13:397–407. [PMC free article] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, et al. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- Yang J, Menshenina J, Cunha G, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J Urol. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiou R, Delmas V, Carmeliet P, Gherardi RK, Barlovatz-Meimon G, et al. The pathophysiology of pelvic floor disorders: evidence from a histomorphologic study of the perineum and a mouse model of rectal prolapse. J Anat. 2001:599–607. doi: 10.1046/j.1469-7580.2001.19950599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.