Figure 1.
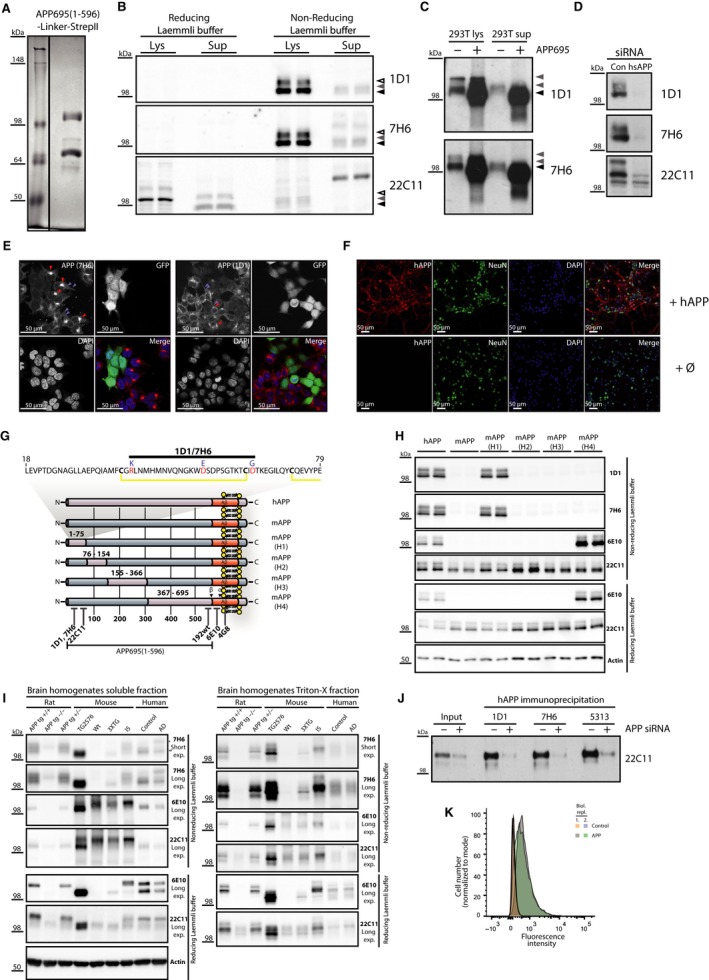
Validation of novel APP‐specific antibodies in different applications. (A) Quality control of hAPP‐linker‐Precission‐StrepII antigen on a Coomassie gel prior to vaccination (Left lane: molecular weight marker, right lane: hAPP‐linker‐Precission‐StrepII). (B) Validation of novel antibodies 1D1 and 7H6 for the detection of endogenous hAPP in HEK293T lysates (Lys) and conditioned supernatant (Sup) under reducing (reducing Laemmli buffer) and nonreducing (nonreducing Laemmli buffer) conditions by Western blot analysis. 1D1 and 7H6 detected a specific band for APP in conditioned media (grey arrowhead) and two bands immature (black arrowhead) and mature APP (empty arrowhead) in cell lysates of HEK293T (293T) cells all above 98 kDa only under nonreducing conditions while 22C11 detected a specific signal in lysates and conditioned media only under reducing conditions. (C) Specificity of the novel antibodies was further validated comparing HEK293T cells (−) with HEK293T cells overexpressing hAPP695 (+). Both antibodies detected endogenous APP751 and APP770 in HEK293T (grey arrowheads) and overexpressed APP695 at a slightly lower molecular weight as a strong increase of the 98 kD bands. Sup: supernatant. (D) Specificity of both antibodies was tested in HEK293T cell lysates with a siRNA‐mediated APP knockdown. APP knockdown was validated with 22C11 which shows additional remaining unspecific bands. 1D1 and 7H6 bands were completely abolished upon APP knockdown. (E) Specificity of both antibodies was tested in immunocytochemistry. HEK293T cells with a lentivirus‐mediated APP knockdown and GFP expression were mixed with wild‐type HEK293T cells and stained with 7H6 and 1D1. Both antibodies show a Golgi (red arrows) and vesicular staining (light blue arrows) which is abolished upon APP knockdown (see GFP‐positive cells; APP red, DAPI blue). (F) Both antibodies were tested for their specificity towards hAPP in primary cortical neurons of mice. Only upon lentiviral overexpression of hAPP, a specific signal could be observed with the antibody 7H6, while in the nontransduced control neurons (Ø), no signal was detected. 1D1 showed the same staining pattern. (G) We identified the epitope of 7H6 and 1D1 creating chimeric APP constructs by the exchange of distinct parts of murine APP for human APP (mAPP‐(H1‐H4)). These constructs were expressed together with murine and hAPP in HEK293T cells. Pink colour indicates hAPP sequence; blue colour indicates murine APP sequence. (H) While 22C11 detected all constructs and 6E10 detected hAPP and mAPP‐H4 under reducing and nonreducing conditions, 7H6 and 1D1 detected only hAPP and the chimeric APP construct mAPP‐H1 under nonreducing conditions which shows that the epitope lies between amino acid 1–75. The proposed binding epitope is depicted above the chimeric APP constructs. (I) 1D1 and 7H6 were tested and compared to 22C11 and 6E10 for their specificity towards hAPP in brain homogenates of wild‐type mice, APP‐transgenic mouse models, an APP‐transgenic rat model and human healthy and AD brains under reducing and nonreducing conditions by Western blot analysis. 22C11 detected a signal in all mice and rats as well as human brains properly only under reducing conditions but not under nonreducing conditions due to the presence of additional background bands. 6E10 properly detected APP only in transgenic animals under reducing conditions but detected additional strong unspecific bands under nonreducing conditions in mouse models which made discrimination between wild‐type and APP‐transgenic mice difficult except for Tg2576 mice which heavily overexpress hAPP. 7H6 only detected a clear signal in transgenic mice and rats. Furthermore, 7H6 detected a shift of APP695 (*) towards the APP770 isoform (**) between healthy and AD human brains which reflects neuronal loss and astrogliosis in AD pathogenesis. (J) Antibodies 7H6 and 1D1 were tested and compared to the polyclonal antibody 5313 for their ability to immunoprecipitate hAPP from conditioned media of HEK293T cells. About 20 μL of directly loaded supernatants of HEK293T cells (input) transfected with a control (−) or an APP‐specific siRNA (+) was compared to immunoprecipitated hAPP of 200 μL medium for each antibody. Specificity of immunoprecipitated material was proven by the hAPP‐specific siRNA‐mediated knockdown. (K) 7H6 antibody was tested for its applicability in FACS. Overexpression of hAPP led to a clearly detectable increase of the 555 signal (shift towards the right). Orange and light blue indicate biological replicates of control cells, and grey and green indicate biological replicates of APP overexpressing cells.
