Abstract
The aim of this study was to explore the relationship between serum magnesium and peripheral nerve function in patients with type 2 diabetes (T2DM). A total of 978 T2DM patients were included in the study. Patients were divided into tertiles according to serum magnesium concentration (low tertile: ≤0.85 mmol/L; medium tertile: 0.85 to 0.92 mmol/L; and high tertile: >0.92 mmol/L). All participants underwent nerve conduction (NC) studies. Composite z scores of conduction velocity, latency, and amplitude were constructed, respectively. The serum magnesium levels were significantly lower in patients with abnormal NC than in those with normal NC (0.87 [0.82, 0.92] vs. 0.88 [0.83, 0.93] mmol/L, P = 0.048). The composite z score of amplitude significantly increased with increasing tertiles of magnesium (−0.60 ± 0.02 vs. −0.57 ± 0.02 vs. −0.48 ± 0.03, P for trend = 0.001). After adjusting for all potential confounders, lower serum magnesium levels were still associated with lower composite z score of amplitude (β = 0.095, P = 0.014). In patients with T2DM, lower serum magnesium levels were significantly associated with lower composite z score of amplitude, indicating magnesium might affect peripheral nerve function through axonal degeneration.
Diabetes is becoming a major public health threat in China1. Despite improvements in the management of diabetes, diabetic peripheral neuropathy (DPN) has become the most commonly reported chronic diabetic complication, affecting up to half of diabetic patients2. DPN causes serious compilations, such as foot ulcers and gangrene, leading to lower limb amputation, all of which reduce the quality of life in diabetic patients3,4. Several pathogenic mechanisms have been reported to be involved in DPN, including microangiopathy, oxidative stress, inflammation, the polyol pathway, glycation, and ligand activation5,6,7,8. However, the underlying pathophysiology of DPN is complex and has not been fully elucidated.
Magnesium is the fourth most abundant cation in the human body. It acts as a co-factor for numerous enzymatic reactions and exerts important roles in many biological processes9,10. Recent studies have demonstrated that low serum magnesium is independently associated with an increased risk of type 2 diabetes (T2DM)11,12,13, cardiovascular disease14, and foot ulcers15. Furthermore, there is increasing evidence that hypomagnesemia is associated with microvascular complications of T2DM, such as nephropathy16,17,18 and retinopathy19,20. The relationship between serum magnesium and DPN remains unclear and controversial, with conflicting results observed regarding the effects of serum magnesium levels on nerve conduction (NC) and the presence of neuropathic pain21,22,23. Therefore, we designed this cross-sectional study to examine the association between serum magnesium levels and peripheral nerve function in patients with T2DM.
Results
Patient characteristics
The demographic and clinical characteristics of patients with normal and abnormal NC are illustrated in Table 1. Among the 978 patients, 746 (76.28%) had normal NC and 232 (23.72%) had abnormal NC. Abnormal NC was significantly associated with older age, longer diabetes duration (all P < 0.001). Besides, HbA1c, systolic blood pressure (SBP), urinary albumin excretion (UAE) and the percentage of anti-hypertensive therapy were significantly higher in the abnormal NC group (all P < 0.05). Interestingly, serum magnesium levels were significantly lower in the abnormal NC group (P = 0.048).
Table 1. Characteristics of patients in the study.
| Variable | Total (N = 978) | Normal NC (N = 746) | Abnormal NC (N = 232) | P value |
|---|---|---|---|---|
| Age (years) | 57.32 ± 12.77 | 56.43 ± 12.97 | 60.18 ± 11.65 | <0.001 |
| Male sex (n, %) | 541 (55.32%) | 410 (54.96%) | 131 (56.47%) | 0.687 |
| Alcohol consumers (n, %) | 177 (18.17%) | 128 (17.23%) | 49 (21.21%) | 0.170 |
| Current smokers (n, %) | 229 (23.44%) | 171 (22.95%) | 58 (25.00%) | 0.520 |
| SBP (mmHg) | 130.64 ± 15.27 | 129.79 ± 14.97 | 133.40 ± 15.96 | 0.002 |
| DBP (mmHg) | 79.17 ± 9.03 | 79.20 ± 9.06 | 79.07 ± 8.95 | 0.844 |
| BMI (kg/m2) | 25.21 (22.85, 27.73) | 25.11 (22.84, 27.51) | 25.39 (23.15, 28.65) | 0.098 |
| Diabetes duration (years) | 8.00 (4.00, 14.00) | 8.00 (3.00, 13.00) | 10.00 (5.00, 15.75) | <0.001 |
| HbA1c (%) | 8.00 (7.10, 9.50) | 7.80 (6.90, 9.30) | 8.75 (7.60, 10.10) | <0.001 |
| Total cholesterol (mmol/L) | 4.59 (4.01, 5.32) | 4.62 (4.04, 5.31) | 4.50 (3.91, 5.36) | 0.555 |
| HDL-c (mmol/L) | 1.02 (0.87, 1.23) | 1.02 (0.87, 1.23) | 1.02 (0.87, 1.21) | 0.793 |
| LDL-c(mmol/L) | 2.80 (2.21, 3.38) | 2.82 (2.26, 3.38) | 2.74 (2.14, 3.39) | 0.361 |
| Triglyceride (mmol/L) | 1.39 (1.01, 1.99) | 1.38 (1.02, 1.97) | 1.42 (1.01, 2.03) | 0.863 |
| Serum creatinine (μmol/L) | 67.00 (55.00, 78.00) | 67.00 (55.00, 78.00) | 68.00 (56.50, 78.00) | 0.505 |
| eGFR (ml · min−1 · 1.73 m−2) | 129.56 (111.69, 149.36) | 130.01 (112.95, 149.75) | 128.18 (106.21, 148.77) | 0.284 |
| UAE (mg/24h) | 9.82 (6.27, 24.60) | 9.32 (6.14, 20.47) | 14.10 (7.08, 43.12) | <0.001 |
| Anti-hypertensive therapy (n, %) | 532 (54.40%) | 385 (51.61%) | 147 (63.36%) | 0.002 |
| Serum magnesium (mmol/L) | 0.88 (0.83, 0.93) | 0.88 (0.83, 0.93) | 0.87 (0.82, 0.92) | 0.048 |
Data were expressed as mean ± standard deviation (SD) for normal distribution variables or as median (25–75th percentiles) for skewed distribution variables. Categorical variables were expressed as numbers (percentage).
NC, nerve conduction; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HbA1c, glycated hemoglobin; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; and UAE, urinary albumin excretion.
Association of serum magnesium levels with NC parameters
Patients were further divided into tertiles according to serum magnesium levels (low tertile: ≤0.85 mmol/L; medium tertile: 0.85 to 0.92 mmol/L, and high tertile: >0.92 mmol/L). As presented in Table 2, patients in the high tertile of serum magnesium were older, had lower levels of body mass index (BMI), HbA1c, estimated glomerular filtration rate (eGFR) and UAE than those in the low and medium tertile (all P < 0.001). Besides, diastolic blood pressure (DBP), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), creatinine, and the percentage of anti-hypertensive therapy were significantly different among the 3 tertiles (all P < 0.05).
Table 2. Characteristics of patients and nerve conduction parameters, according to tertiles of serum magnesium.
| Variable | Serum magnesium |
P value | ||
|---|---|---|---|---|
| Low tertile (N = 366) | Medium tertile (N = 340) | High tertile (N = 272) | ||
| Serum magnesium (mmol/L) | ≤0.85 | 0.85–0.92 | >0.92 | |
| Age (years) | 54.98 ± 13.27 | 58.40 ± 12.69 | 59.13 ± 11.68 | <0.001 |
| Male sex (n, %) | 190 (51.91%) | 194 (57.06%) | 157 (57.72%) | 0.250 |
| Alcohol consumers (n, %) | 64 (17.53%) | 65 (19.23%) | 48 (17.71%) | 0.822 |
| Current smokers (n, %) | 78 (21.31%) | 90 (26.55%) | 61 (22.43%) | 0.234 |
| SBP (mmHg) | 130.89 ± 14.81 | 131.28 ± 15.36 | 129.50 ± 15.77 | 0.333 |
| DBP (mmHg) | 79.36 ± 8.47 | 80.20 ± 9.55 | 77.63 ± 8.94 | 0.002 |
| BMI (kg/m2) | 25.64 (23.24, 28.48) | 25.20 (22.91, 27.66) | 24.22 (22.41, 26.68) | <0.001 |
| Diabetes duration (years) | 9.00 (4.00, 14.00) | 8.00 (4.00, 13.00) | 8.00 (3.00, 14.00) | 0.344 |
| HbA1c (%) | 8.60 (7.30, 10.00) | 8.20 (7.20, 9.50) | 7.40 (6.70, 8.70) | <0.001 |
| Total cholesterol (mmol/L) | 4.55 (4.00, 5.32) | 4.65 (4.06, 5.41) | 4.60 (3.99, 5.26) | 0.495 |
| HDL-c (mmol/L) | 0.99 (0.86, 1.17) | 1.02 (0.88, 1.23) | 1.07 (0.89, 1.29) | 0.006 |
| LDL-c (mmol/L) | 2.79 (2.14, 3.35) | 2.87 (2.32, 3.41) | 2.71 (2.17, 3.32) | 0.221 |
| Triglyceride (mmol/L) | 1.50 (1.09, 2.21) | 1.37 (1.00, 1.99) | 1.29 (0.97, 1.82) | 0.002 |
| Serum creatinine (μmol/L) | 64.00 (52.00, 77.00) | 66.00 (56.00, 76.00) | 70.00 (59.00, 81.00) | <0.001 |
| eGFR (ml · min−1 · 1.73 m−2) | 134.23 (114.51, 158.77) | 131.49 (113.33, 148.26) | 122.92 (104.84, 139.82) | <0.001 |
| UAE (mg/ 24h) | 13.06 (7.00, 34.40) | 9.50 (6.29, 24.86) | 7.50 (5.63, 14.95) | <0.001 |
| Anti-hypertensive therapy (n, %) | 218 (59.56%) | 187 (55.00%) | 127 (46.69%) | 0.005 |
| Motor median CV (m/s) | 53.85 (51.20, 56.81) | 53.75 (51.20, 56.63) | 54.45 (51.33, 58.10) | 0.105 |
| Motor median amplitude (mv) | 5.95 (4.28, 7.63) | 5.80 (4.10, 7.50) | 6.40 (4.50, 8.20) | 0.130 |
| Motor median latency (ms) | 3.45 (3.10, 3.90) | 3.50 (3.10, 3.80) | 3.40 (3.00, 3.80) | 0.201 |
| Motor ulnar CV (m/s) | 58.70 (54.30, 62.90) | 58.30 (54.51, 62.18) | 59.00 (54.79, 63.20) | 0.412 |
| Motor ulnar amplitude (mv) | 4.40 (3.58, 5.70) | 4.50 (3.50, 5.48) | 4.75 (3.60, 6.00) | 0.164 |
| Motor ulnar latency (ms) | 2.45 (2.20, 2.70) | 2.40 (2.20, 2.70) | 2.40 (2.20, 2.60) | 0.125 |
| Motor tibial CV (m/s) | 42.90 (40.28, 47.10) | 43.31 (40.70, 47.90) | 43.78 (41.50, 47.10) | 0.064 |
| Motor tibial amplitude (mv) | 6.10 (3.90, 8.13) | 6.40 (4.13, 8.88) | 6.60 (4.63, 9.38) | 0.046 |
| Motor tibial latency (ms) | 3.60 (3.20, 4.10) | 3.50 (3.20, 4.00) | 3.50 (3.10, 4.10) | 0.517 |
| Sensory median CV (m/s) | 56.00 (50.78, 61.93) | 56.00 (50.08, 61.13) | 55.20 (51.63, 60.90) | 0.836 |
| Sensory median amplitude (mv) | 9.30 (6.10, 13.00) | 9.00 (5.75, 13.93) | 10.00 (6.40, 15.00) | 0.124 |
| Sensory median latency (ms) | 2.50 (2.30, 2.80) | 2.50 (2.20, 2.80) | 2.50 (2.30, 2.70) | 0.791 |
| Sensory ulnar CV (m/s) | 58.00 (52.40, 62.50) | 57.11 (52.30, 61.90) | 58.00 (52.20, 62.50) | 0.926 |
| Sensory ulnar amplitude (mv) | 7.95 (5.20, 10.50) | 8.30 (5.90, 11.60) | 8.35 (5.80, 11.00) | 0.044 |
| Sensory ulnar latency (ms) | 2.10 (1.90, 2.30) | 2.10 (1.92, 2.40) | 2.10 (1.90, 2.38) | 0.792 |
| Sensory sural CV (m/s) | 46.20 (42.38, 51.55) | 46.95 (42.90, 52.08) | 46.85 (42.90, 50.70) | 0.745 |
| Sensory sural amplitude (mv) | 10.00 (5.88, 15.00) | 10.20 (6.60, 16.00) | 11.00 (7.03, 18.80) | 0.012 |
| Sensory sural latency (ms) | 1.90 (1.50, 2.40) | 2.00 (1.60, 2.50) | 1.90 (1.50, 2.40) | 0.183 |
Data were expressed as mean ± standard deviation (SD) for normal distribution variables or as median (25–75th percentiles) for skewed distribution variables. Categorical variables were expressed as numbers (percentage).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HbA1c, glycated hemoglobin; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; UAE, urinary albumin excretion; and CV, conduction velocity.
All individual nerve conduction studies (NCS) parameters were analyzed firstly. Significant differences were observed in amplitude for motor tibial (P = 0.046), sensory ulnar (P = 0.044) and sensory sural nerves (P = 0.012) among the three tertiles of serum magnesium (Table 2). However, there was no significant difference in the conduction velocity (CV) and latency for all tested nerves. In addition, there was no significant difference of CV, amplitude and latency for peroneal nerve among serum magnesium tertiles (Supplementary Table S1).
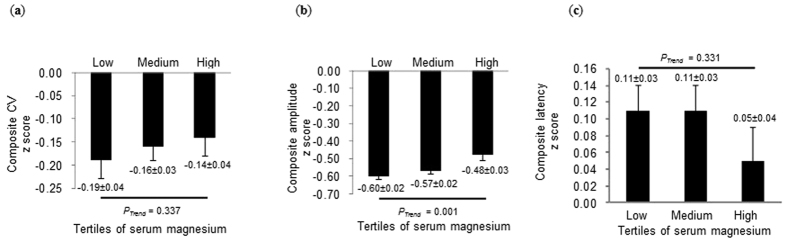
Next, composite z scores of CV, latency, and amplitude were calculated, respectively. The results showed that the composite z score of amplitude significantly increased with increasing tertiles of magnesium (−0.60 ± 0.02 vs. −0.57 ± 0.02 vs. −0.48 ± 0.03, P for trend = 0.001; Fig. 1b). In addition, post-hoc analysis using Bonferroni correction showed significant differences in the composite z score of amplitude between the medium and high tertile (P = 0.047) as well as between the low and high tertile (P = 0.002). Notably, the result regarding the composite z score of amplitude was consistent with that of amplitude analysis in individual nerves. However, no significant trends were observed among tertiles of magnesium with respect to the composite z scores of both CV (P for trend = 0.337) and latency (P for trend = 0.331).
Figure 1. Composite z scores of nerve conduction parameters, according to tertiles of serum magnesium.
Results were represented as mean ± standard error (s.e.m.). (a) Composite z score of conduction velocity (CV) across serum magnesium tertiles; (b) Composite z score of amplitude across serum magnesium tertiles; (c) Composite z score of latency across serum magnesium tertiles.
Multiple linear regression analysis showed that serum magnesium was still positively correlated with the composite z score of amplitude (β = 0.102, P = 0.007; Table 3), after adjusting for age, sex, diabetes duration, HbA1c level, anti-hypertensive therapy, SBP, DBP, UAE (model 1). Given that serum magnesium has been shown to be negatively correlated with eGFR14,24 and our results were also in accordance with this observation (r = −0.19, P<0.001), eGFR was additionally included for adjustment (model 2). We found that the association of serum magnesium with the composite z score of amplitude (β = 0.095, P = 0.014) remained significant.
Table 3. Association of serum magnesium levels with nerve conduction parameters after adjustments.
| Variable | Serum magnesium | |
|---|---|---|
| β | P value | |
| Model 1 | ||
| Composite z score of CV | 0.010 | 0.792 |
| Composite z score of amplitude | 0.102 | 0.007 |
| Composite z score of latency | −0.025 | 0.483 |
| Model 2 | ||
| Composite z score of CV | 0.004 | 0.918 |
| Composite z score of amplitude | 0.095 | 0.014 |
| Composite z score of latency | −0.023 | 0.526 |
CV, conduction velocity.
Model 1: adjusted for age, sex, diabetes duration, HbA1c, anti-hypertensive therapy, systolic blood pressure, diastolic blood pressure, urinary albumin excretion.
Model 2: Model 1 + estimated glomerular filtration rate.
Discussion
Serum magnesium has a close relationship with T2DM11,12. It has been shown that lower serum magnesium levels are significantly associated with increased risk of T2DM13 and with various complications of diabetes, including cardiovascular disease14, nephropathy16,17,18,25, retinopathy19,20, and foot ulcers15. However, data regarding the relationship between serum magnesium and DPN are limited and controversial. Previous research has shown that magnesium supplementation could improve both NC in type 1 diabetic patients21 and symptoms of neuropathy in streptozocin-induced diabetic (STZ-D) rats22. In contrast, Hyassat et al. found no association between hypomagnesemia and neuropathy when the diagnosis of neuropathy was based on neuropathic symptoms or the presence of an abnormality of NC23.
In this study, we performed NCS in all patients, which is an accurate, sensitive, and reproducible method for evaluating DPN26,27. Additionally, composite z scores of NCS parameters were constructed for six nerves, including motor nerves (median, ulnar, and tibial) and sensory nerves (median, ulnar, and sural)28. We found that serum magnesium levels were independently associated with the amplitude, but not with CV and latency. Interestingly, it is well established that low amplitude is an indicator of axonal degeneration, whereas decreased CV and prolonged latency are useful markers for demyelination29. It is plausible to postulate that serum magnesium levels might affect peripheral nerve function through axonal degeneration.
Magnesium exerts wide-range effects on many biological processes. First of all, increasing evidences indicated that magnesium can not only decrease tissue susceptibility to oxidative damage, but also has indirect antioxidant capacity30,31,32. Parvizi et al. found that treatment of STZ-D rats with MgSO4 can attenuate oxidative stress in the renal tissue as indicated by decreased levels of malondialdehyde33. Additionally, several studies have demonstrated that low serum magnesium concentration is closely associated with increased inflammation34,35. Furthermore, it has been reported that magnesium can increase intracellular inositol concentrations by enhancing the affinity of transport system for inositol, thus inhibiting further damage of the nervous system32,36. Although the multifactorial pathogenesis of DPN is still poorly understood, oxidative stress, inflammation and decreased intracellular inositol concentrations contribute to the progression of DPN5,8,32,37. In addition, myo-inositol may be at least partially responsible for the axonal degeneration38. Further studies are warranted to reveal the direct effects of low serum magnesium levels on axonal degeneration. Notably, in non-diabetic subjects, we did not observe any association between serum magnesium levels and the composite z scores of all NCS parameters, suggesting that magnesium will only affect NC function in diabetic patients.
In summary, our data showed that lower serum magnesium levels were significantly associated with lower composite z score of amplitude in patients with T2DM, indicating low serum magnesium levels might affect peripheral nerve function through axonal degeneration. Our finding suggested that low serum magnesium levels may underlie many of the pathophysiologic features of DPN. Further studies will provide a novel prospective strategy for DPN.
Methods
Study population
A total of 978 T2DM patients were recruited from the Shanghai Diabetes Institute Inpatient Database of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital between April 2013 and August 2014. Inclusion criteria include the following: (1) established T2DM diagnosed according to the 1999 WHO definition (fasting plasma glucose ≥7.0 mmol/L and/or 2-h plasma glucose ≥11.1 mmol/L); (2) valid data of NCS. Exclusion criteria included: (1) missing data on age, sex, diabetes duration, and serum magnesium; (2) histories of persistent diarrhea or vomiting, progressive malignancy and severe renal insufficiency as defined by an eGFR <60 ml·min−1·1.73 m−2; (3) current use of high-dose (>40 mg/day) diuretics and/or magnesium supplementation; (4) histories of diseases that could affect NC (i.e., Guillain–Barre syndrome, chronic inflammatory demyelinating polyneuropathy, or carpal tunnel syndrome, etc.).
This study was conducted in accordance with the Declaration of Helsinki II and was approved by the institutional review board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from each participants.
Clinical and laboratory analysis
Weight and height were measured with a standardized method by the same physician. BMI was calculated as the body weight (kg) divided by the square of the height (m). Information of alcohol use and smoking status were obtained using a standardized questionnaire. Participants consuming alcohol on a regular basis (≥30 g per week) for more than 1 year were defined as alcohol consumers1. Subjects who smoked at least one cigarette per day for over 6 months were defined as current smokers39. SBP and DBP were calculated as the average value of three measurements taken at 3 min intervals using a mercury sphygmomanometer. Information regarding anti-hypertensive therapy (i.e., Angiotensin-converting enzyme inhibitor, Angiotensin II receptor blocker, Calcium channel blocker, and β-blocker etc.) were obtained from all participants’ medical records.
Blood samples were collected after an overnight fast of 8–10 h. Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (HLC-723 G7, Tosoh, Japan). Serum magnesium levels were measured by the xylidyl blue method (Hitachi 7600 analyzer). Serum creatinine, total cholesterol (TC), HDL-c, low-density lipoprotein cholesterol (LDL-c), and TG were determined enzymatically (Hitachi 7600 chemical analyzer). The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula developed for the Chinese population: eGFR (ml·min−1 ·1.73 m−2) = 186 × (serum creatinine in μmol/L × 0.011)−1.154 × (age in years)–0.203 × (0.742 if female) × (1.233 if Chinese)40. UAE was obtained from at least two 24-h urine samples and determined as the mean of 24-h urine collections during the period of hospitalization.
Nerve conduction studies
Peripheral nerve function was evaluated by NCS with the use of the EMG Myto, EBNeuro (ESAOTE, Florence, Italy), which has been described previously41. Briefly, NCS were performed by experienced electrophysiologists who were unaware of the laboratory results. During testing, temperatures were maintained at ≥35 °C for upper extremities and ≥32 °C for lower extremities. Motor nerve studies measured CV, compound muscle action potential (CMAP) amplitude, and distal latency in the median, ulnar, and tibial nerves. Sensory nerve studies contained the CV, sensory nerve action potential (SNAP) amplitude, and onset latency in the median, ulnar, and sural nerves. We then compared all obtained data with reference values from our laboratory. Throughout the study, all data were reviewed by the reading site to ensure overall quality. Abnormal NC was defined by abnormality of one or more parameters in two or more tested anatomical nerves42. Composite z scores of CV, amplitude, and latency were constructed as previously described41,43.
F-wave analysis
Supramaximal percutaneous stimuli at the degree of 1 Hz were given to peroneal, tibial, median and ulnar nerves for ten times, respectively. F-wave was recorded at the extensor digitorum brevis, abductor hallucis muscle, abductor pollicis brevis, and abductor digiti minimi by means of the EMG Myto, EBNeuro (ESAOTE, Florence, Italy). Variables analyzed included F-wave minimum latency and persistence. F-wave persistence was defined as the number of the F-responses obtained with ten stimuli. The details of F-wave analysis were shown in Supplementary Table S2 and S3.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) for normal distribution variables or as median (25–75th percentiles) for skewed distribution variables. Categorical variables were presented as numbers (percentage). Differences between the normal NC group and abnormal NC group were evaluated using the Student’s t-test or Mann–Whitney U test for continuous variables and the chi-squared test for categorical variables. Differences among tertiles of serum magnesium were analysed by one-way ANOVA or the Kruskal-Wallis test, as well as the chi-squared test for categorical values. Bonferroni correction was used for the post-hoc analyses. Data with a skewed distribution (including serum magnesium, and eGFR) were logarithmically transformed before analysis. Multivariate linear regression analysis was used to assess the independent associations of serum magnesium with NCS parameters after adjusting for covariates. All P values were two-sided, and values of P < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 19 (SPSS, Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Chu, C. et al. Low serum magnesium levels are associated with impaired peripheral nerve function in type 2 diabetic patients. Sci. Rep. 6, 32623; doi: 10.1038/srep32623 (2016).
Supplementary Material
Acknowledgments
We thank all clinicians, nurses, and technicians who contributed to this work. The study was supported by grants from the Innovation Foundation of Translational Medicine of Shanghai Jiao Tong University School of Medicine–Project of Precision Medicine (No. 15ZH4006) and the Programs of the National Natural Science Foundation of China (81570808).
Footnotes
The authors declare no competing financial interests.
Author Contributions C.W. and W.J. contributed to the planning of the study. C.C., W.Z. and Y.Z. wrote the manuscript, analyzed and interpreted data. L.L., J.L. and L.J. performed research. All authors reviewed the manuscript and approved the final draft.
References
- Yang W. et al. Prevalence of diabetes among men and women in China. N Engl J Med 362, 1090–1101, 10.1056/NEJMoa0908292 (2010). [DOI] [PubMed] [Google Scholar]
- Tesfaye S. et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33, 2285–2293, 10.2337/dc10-1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R. A. Which test for diagnosing early human diabetic neuropathy? Diabetes 63, 2206–2208, 10.2337/db14-0492 (2014). [DOI] [PubMed] [Google Scholar]
- Boulton A. J., Vileikyte L., Ragnarson-Tennvall G. & Apelqvist J. The global burden of diabetic foot disease. Lancet 366, 1719–1724, 10.1016/s0140-6736(05)67698-2 (2005). [DOI] [PubMed] [Google Scholar]
- Pasnoor M., Dimachkie M. M., Kluding P. & Barohn R. J. Diabetic neuropathy part 1: overview and symmetric phenotypes. Neurol Clin 31, 425–445, 10.1016/j.ncl.2013.02.004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C. et al. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes 57, 1002–1017, 10.2337/db07-0339 (2008). [DOI] [PubMed] [Google Scholar]
- Zochodne D. W. Diabetic polyneuropathy: an update. Curr Opin Neurol 21, 527–533, 10.1097/WCO.0b013e32830b84cb (2008). [DOI] [PubMed] [Google Scholar]
- Vincent A. M., Russell J. W., Low P. & Feldman E. L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25, 612–628, 10.1210/er.2003-0019 (2004). [DOI] [PubMed] [Google Scholar]
- Pham P. C., Pham P. M., Pham S. V., Miller J. M. & Pham P. T. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2, 366–373, 10.2215/cjn.02960906 (2007). [DOI] [PubMed] [Google Scholar]
- Saris N. E., Mervaala E., Karppanen H., Khawaja J. A. & Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta 294, 1–26 (2000). [DOI] [PubMed] [Google Scholar]
- Dong J. Y., Xun P., He K. & Qin L. Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 34, 2116–2122, 10.2337/dc11-0518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales C. H. & Pedrosa Lde F. Magnesium and diabetes mellitus: their relation. Clin Nutr 25, 554–562, 10.1016/j.clnu.2006.03.003 (2006). [DOI] [PubMed] [Google Scholar]
- Fang C. et al. Association of Serum Magnesium Level with Odds of Prediabetes and Diabetes in a Southern Chinese Population: a Prospective Nested Case-Control Study. Biol Trace Elem Res 172, 307–314, 10.1007/s12011-015-0594-y (2016). [DOI] [PubMed] [Google Scholar]
- Wang S. et al. Serum electrolyte levels in relation to macrovascular complications in Chinese patients with diabetes mellitus. Cardiovasc Diabetol 12, 146, 10.1186/1475-2840-12-146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moran M. & Guerrero-Romero F. Low serum magnesium levels and foot ulcers in subjects with type 2 diabetes. Arch Med Res 32, 300–303 (2001). [DOI] [PubMed] [Google Scholar]
- Corsonello A. et al. Serum ionized magnesium levels in type 2 diabetic patients with microalbuminuria or clinical proteinuria. Am J Nephrol 20, 187–192, doi: 13582 (2000). [DOI] [PubMed] [Google Scholar]
- Pham P. C. et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 63, 429–436 (2005). [DOI] [PubMed] [Google Scholar]
- Pham P. C. et al. The link between lower serum magnesium and kidney function in patients with diabetes mellitus Type 2 deserves a closer look. Clin Nephrol 71, 375–379 (2009). [DOI] [PubMed] [Google Scholar]
- de Valk H. W., Hardus P. L., van Rijn H. J. & Erkelens D. W. Plasma magnesium concentration and progression of retinopathy. Diabetes Care 22, 864–865 (1999). [DOI] [PubMed] [Google Scholar]
- Kundu D. et al. Serum magnesium levels in patients with diabetic retinopathy. J Nat Sci Biol Med 4, 113–116, 10.4103/0976-9668.107270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen W., Bouten A., De Leeuw I. & De Block C. Are low magnesium levels in type 1 diabetes associated with electromyographical signs of polyneuropathy? Magnes Res 13, 197–203 (2000). [PubMed] [Google Scholar]
- Rondon L. J. et al. Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. J Physiol 588, 4205–4215, 10.1113/jphysiol.2010.197004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyassat D., Al Sitri E., Batieha A., El-Khateeb M. & Ajlouni K. Prevalence of Hypomagnesaemia among Obese Type 2 Diabetic Patients Attending the National Center for Diabetes, Endocrinology and Genetics (NCDEG). Int J Endocrinol Metab 12, e17796, 10.5812/ijem.17796 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham P. C., Pham P. M. & Pham P. T. Patients with diabetes mellitus type 2 and hypomagnesemia may have enhanced glomerular filtration via hypocalcemia. Clin Nephrol 78, 442–448, 10.5414/cn107525 (2012). [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y. et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care 35, 1591–1597, 10.2337/dc12-0226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman A. et al. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS One 8, e58783, 10.1371/journal.pone.0058783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins B. A. & Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin Neurophysiol 114, 1167–1175 (2003). [DOI] [PubMed] [Google Scholar]
- Dyck P. J. et al. Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve 27, 202–210, 10.1002/mus.10320 (2003). [DOI] [PubMed] [Google Scholar]
- Chung T., Prasad K. & Lloyd T. E. Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am 24, 49–65, 10.1016/j.nic.2013.03.023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol 3, 41–52 (2004). [DOI] [PubMed] [Google Scholar]
- Mazur A. et al. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys 458, 48–56, 10.1016/j.abb.2006.03.031 (2007). [DOI] [PubMed] [Google Scholar]
- Hasanein P. et al. Oral magnesium administration prevents thermal hyperalgesia induced by diabetes in rats. Diabetes Res Clin Pract 73, 17–22, 10.1016/j.diabres.2005.12.004 (2006). [DOI] [PubMed] [Google Scholar]
- Parvizi M. R. et al. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J Diabetes Metab Disord 13, 84, 10.1186/s40200-014-0084-3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F. H. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev 68, 333–340, 10.1111/j.1753-4887.2010.00293.x (2010). [DOI] [PubMed] [Google Scholar]
- Guerrero-Romero F. & Rodriguez-Moran M. Relationship between serum magnesium levels and C-reactive protein concentration, in non-diabetic, non-hypertensive obese subjects. Int J Obes Relat Metab Disord 26, 469–474 (2002). [DOI] [PubMed] [Google Scholar]
- Grafton G., Bunce C. M., Sheppard M. C., Brown G. & Baxter M. A. Effect of Mg2+ on Na(+)-dependent inositol transport. Role for Mg2+ in etiology of diabetic complications. Diabetes 41, 35–39 (1992). [DOI] [PubMed] [Google Scholar]
- Sandireddy R., Yerra V. G., Areti A., Komirishetty P. & Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. 2014, 674987, 10.1155/2014/674987 (2014). [DOI] [PMC free article] [PubMed]
- Mayer J. H. & Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia 25, 433–438 (1983). [DOI] [PubMed] [Google Scholar]
- Shi L. et al. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS One 8, e77919, 10.1371/journal.pone.0077919 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. C. et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17, 2937–2944, 10.1681/asn.2006040368 (2006). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Serum albumin is associated with peripheral nerve function in patients with type 2 diabetes. Endocrine 50, 397–404, 10.1007/s12020-015-0588-8 (2015). [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Carter R. E. & Litchy W. J. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 44, 340–345, 10.1002/mus.22074 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhamidi K., Dahlin L. B., Boman K. & Rolandsson O. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia 54, 3143–3149, 10.1007/s00125-011-2303-5 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.