Fig. 1. Oxygen regulation of erythrocyte membrane protein interactions.
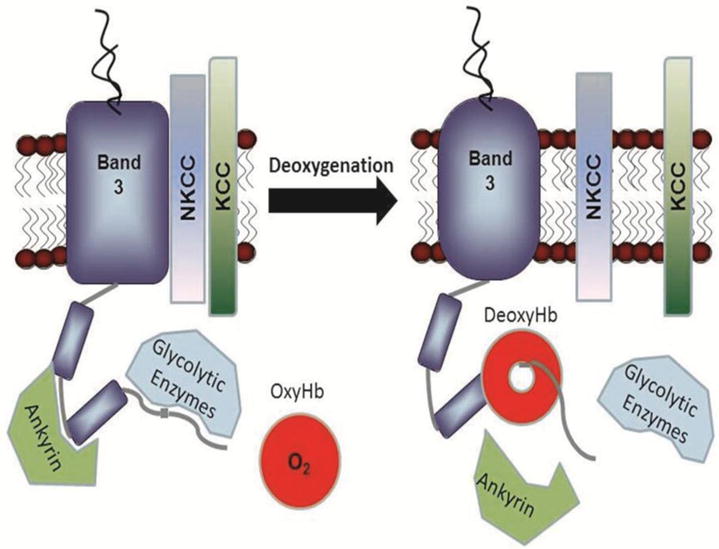
Glucose metabolism11, cation transport1, ATP release7 into circulation and ankyrin binding34 have all been reported to be oxygen-dependent processes in erythrocytes. Because the cytoplasmic domain of band 3 (cdb3) constitutes the only known Hb binding site on the erythrocyte membrane and since the Hb-cdb3 interaction is strongly O2-dependent, many if not all oxygen-regulated pathways in erythrocytes have been postulated to depend on the reversible association of deoxyHb with cdb317. This sketch depicts the binding of deoxyHb to cdb3 and the consequent displacement of several cdb3-associated proteins. Published crystal structure data reveal that deoxyHb binds cdb3 like a “donut on a string”, with the NH2-terminus of cdb3 extending 1.8 nm into the central cavity of deoxyHb51.
