Fig. 6. Measurement of the effect of oxygen on murine cdb3-eGFP binding to murine hemoglobin by FRET.
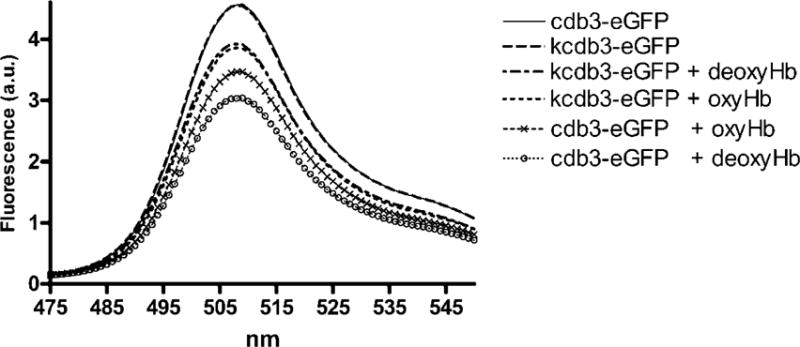
0.5μM of cdb3 was incubated with 1μM Hb under atmospheric conditions, and the emission spectrum of the solution was recorded. This same solution was then deoxygenated under a stream of humidified argon, and the emission spectrum of the solution was again recorded. Deoxygenation was confirmed by monitoring the absorbance of the solution. The kidney spliceoform of cdb3 (kcdb3) was used as a negative control, because it lacks the entire NH2-terminus of cdb3 (residues 1–79) required for deoxyHb binding.
