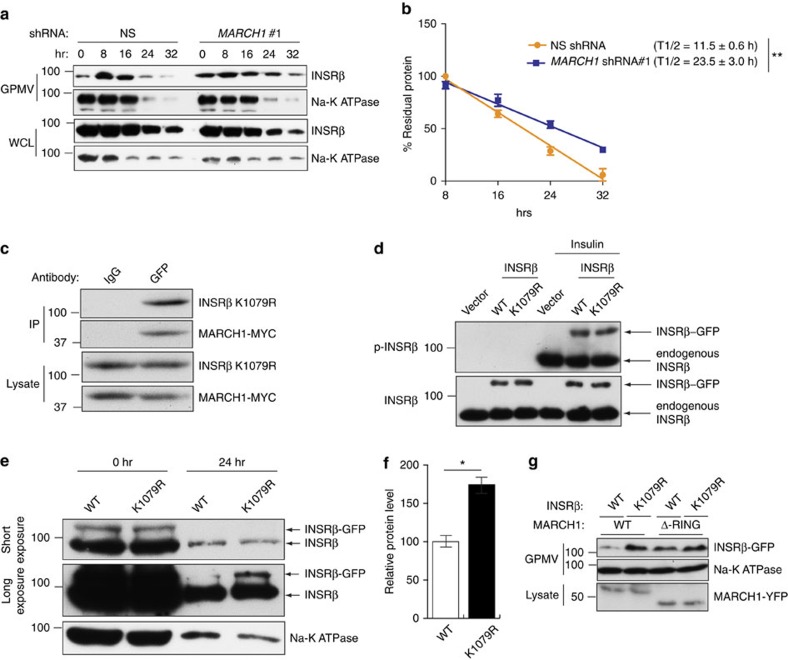
Figure 9. MARCH1 ubiquitination of INSRβ Lys1079 controls INSRβ membrane stability.
(a) Surface INSRβ content in GPMVs from HeLa cells expressing the indicated shRNAs and treated with cycloheximide (see also Supplementary Fig. 7a). (b) Analysis of surface INSRβ half-life, normalized to Na-K ATPase intensity (n=3). (c) Co-immunoprecipitation of INSRβ K1079R-GFP and MARCH1-MYC from HeLa cells expressing both proteins. (d) Immunoblot analysis of INSRβ phosphorylation in HeLa cells expressing vector, INSR-GFP WT or INSR K1079R-GFP with or without insulin stimulation. (e) Immunoblot analysis of INSRβ and Na-K ATPase surface stability in GPMVs of HeLa cells expressing wild-type or K1079R INSR and treated with cycloheximide. Short (top) and long (middle) exposures of INSRβ blots are shown. (f) Densitometric quantitation (n=3) of bands labelled as INSRβ-GFP in e. (g) HeLa cells were transfected with wild-type or K1079R mutant INSR-GFP and either wild-type or ΔRING MARCH1-YFP constructs. GPMVs were analysed for INSRβ and Na-K ATPase and lysates were analysed for MARCH1-YFP by immmunoblot analysis. Data are mean±s.e.m. In (e), n=3 biological replicates. In all panels, *P<0.05, comparisons by t-test.