Abstract
Background:
Bacteriuria and urinary tract infections are common sequelae of benign prostatic hyperplasia (BPH). Thus, the knowledge of urine bacteriology in men with symptomatic BPH in our environment may play a complementary role in management.
Objectives:
To determine the incidence of bacteriuria and the antibiotic sensitivity pattern of bacterial isolates in cultured urine samples of men with symptomatic BPH.
Patients and Methods:
This was a 1 year prospective study. All patients who presented with lower urinary tract symptoms due to BPH and who met the inclusion criteria were studied. Urine samples were obtained from the patients for microscopy, culture, and sensitivity following standard protocol.
Results:
Ninety-four patients were studied. The age range was 53–80 years with a mean of 65.5 ± 7.8 years. Bacterial isolates were noted in 42 (44.7%) patients. Six of these had two different species of bacterial organisms isolated. Escherichia coli noted in 20 (47.6%) specimens was the most common organism isolated while the least common, Providencia species, was noted in 1 (2.4%). The bacterial isolates were mostly sensitive to imipenem, meropenem, and nitrofurantoin, but showed greater resistance to cefuroxime, gentamicin, and ofloxacin. There was no significant difference between the means for age (P = 0.80), duration of symptoms (P = 0.09), and prostate size (P = 0.52) in the patients with and those without bacteriuria.
Conclusion:
Bacteriuria is a common finding in patients with symptomatic BPH in our setting. The bacterial isolates showed high level of resistance to oral cephalosporins and fluoroquinolones. There is a need to update guidelines in empiric use of antibiotics in this group of patients.
KEYWORDS: Bacteriuria, benign prostatic hyperplasia, urinary tract infection
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a common condition in the aging male population.[1] Symptomatic BPH, a cause of significant morbidity in affected males, is characterized by both storage and voiding lower urinary tract symptoms with significant impairment in the quality of life.[2,3]
Affected individuals are prone to the development of bacteriuria as a result of incomplete bladder emptying, urinary stasis, and urethral instrumentation such as cystoscopy and catheterization.[4] A reduction in the concentration of zinc-associated antimicrobial factor and increasing alkalinity of prostatic fluid with aging may also encourage bacterial colonization of the urinary tract in these patients.[5] Some cases of bacteriuria may progress to established urinary tract infection (UTI) if host's defense mechanisms are overcome.[4]
The reported incidence of bacteriuria in men with symptomatic BPH prior to prostatectomy varies with different studies.[6,7] Pourmand et al.[7] noted a preoperative rate of 15% which was reduced to 3.3% after prostatectomy following antibiotic administration. The presence of bacteriuria prior to surgical intervention has been shown to increase the risk of postoperative infective complications such as UTI and wound infection.[8]
Thus, the knowledge of urine bacteriology as well as the antibiotic susceptibility pattern prevailing in the locality at a given period may play a complementary role in the management of patients with BPH. It is expected that such knowledge will enable better antibiotic selection in prophylactic and empirical treatment of UTI during management.
Therefore, the study was aimed at defining the prevailing bacteriology of urine in men with symptomatic BPH in our environment.
PATIENTS AND METHODS
The study, prospective and descriptive in nature, was done at the urology unit of the University of Benin Teaching hospital over a 1 year period (March 2013 – February 2014). Male patients who were referred to the unit on account of lower urinary tract symptoms due to BPH formed the study population. Ninety-four patients who met the inclusion criteria within the period were studied.
Inclusion criteria
Storage and voiding lower urinary tract symptoms due to BPH
Informed consent.
Exclusion criteria
Patients on indwelling catheter
Recent antibiotic use
Immunocompromised state
Uncontrolled diabetes mellitus (fasting blood sugar ≥120 mg/dl)
Steroid therapy.
Methods
Following informed consent, a proforma was opened for each patient to record demographic characteristics, symptoms, and urine microbiology outcome. The size of the prostate on abdominal ultrasonography, as well as the prostate-specific antigen value, was recorded for all patients. Postvoid residual urine volume was recorded only in patients without acute retention of urine. Clean catch mid-stream urine specimens were collected from the patients after evaluation in the outpatient department's specimen collection center. Patients who presented in acute retention of urine had catheter specimens collected at the accident and emergency unit at the time of catheterization for relief of retention. All specimens were sent to the medical microbiology unit and processed within 2 h of collection.
Methodology for specimen collection, transport, and processing
Urine specimens were collected from the patients into a sterile universal container and transported to the microbiology laboratory. The specimens were subjected to microscopy, culture, and sensitivity. The spun deposits of urine centrifuged at 500-1000 g for 5 min were examined microscopically for the presence of bacteria, white blood cells (WBCs), casts, and crystals. Pyuria referring to the presence of ≥10 WBCs per high power field was noted. Specimens were inoculated unto cystine lactose electrolyte deficient agar and blood agar plates, and incubated aerobically at 35-37°C for 24 h. A bacterial count of ≥104 CFU/ml of urine was considered significant. Identification of isolated colonies was based on colony appearance on agar, microscopy of Gram-stained smears, and standard biochemical procedures. Identified isolates were subjected to antimicrobial sensitivity testing using the modified Kirby Bauer disc diffusion method as specified by the clinical laboratory standard institute guidelines.[9] The species of bacteria isolated as well as their antibiotic susceptibility patterns were recorded in the proforma.
Data analysis
Data were presented in the form of tables, frequencies, and percentages. Statistical analysis was done using GraphPad (Graphpad Software Inc, Lajolla, CA 92037, USA) 2014 package. Comparison of the means of continuous variables between patients with and those without bacteriuria was done using unpaired Student's t-test. P < 0.05 was considered significant.
RESULTS
A total of 94 patients were studied. The age range of the patients was 53–80 years with a mean of 65.5 ± 7.8 years. The duration of lower urinary tract symptoms ranged between 2 and 60 months (mean, 21.2 ± 21.1 months) whereas the prostate size on abdominal ultrasonography ranged between 33 and 255 g (mean, 92.3 ± 54.0 g).
Mid-stream urine specimen was obtained in 60 patients whereas catheter urine specimen was obtained in 34 patients at the point of relief of acute urinary retention. Forty-two (44.7%) patients had positive urine cultures. These were recorded in 29 mid-stream and 13 catheter urine specimens (P = 0.39). In six of these, two different species of bacteria organisms were isolated whereas single species was isolated in the remaining 36 specimens [Table 1]. Six patients with bacteriuria had associated dysuria and pyuria in addition to the lower urinary tract symptoms of BPH. Escherichia coli noted in 20 (47.6%) culture-positive specimens was the most common organism isolated whereas the least, Providencia species, was noted in 1 (2.4%). There was no significant difference between the means for age (65.2 vs. 65.7 years, P = 0.80), duration of symptoms (26.8 vs. 15.5 months, P = 0.09), and prostate size (97.5 vs. 87.2 g, P = 0.52) in the patients with and those without bacteriuria. The postvoid residual urine volume in the 60 patients without acute retention ranged from 5 to 1000 ml with a mean of 178.72 ± 185.28 ml. There was no significant difference in the mean residual volume of patients with and those without bacteriuria (P = 0.29). The bacterial isolates on susceptibility testing were mostly sensitive to imipenem (90.5%), meropenem (88.9%), and nitrofurantoin (85.7%) [Figure 1]. Antibiotics to which the isolates were mostly resistant included cefuroxime (70%), gentamicin (73.7%), and ofloxacin (69.7%) [Figure 2]. E. coli isolates showed resistance to the commonly prescribed antibiotics; amoxicillin/clavulanate (90%), ciprofloxacin (75%), gentamicin (60%), and cefuroxime (60%).
Table 1.
Frequency of bacterial isolates
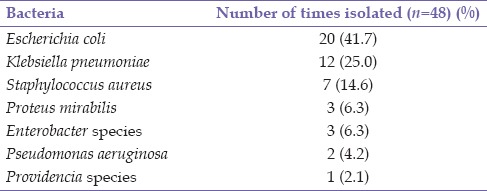
Figure 1.
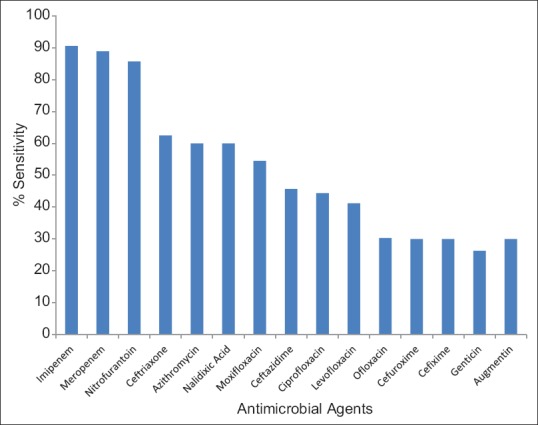
Antimicrobial sensitivity pattern
Figure 2.
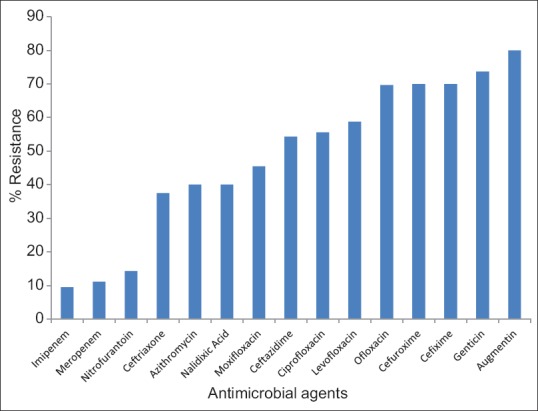
Antimicrobial resistance pattern
DISCUSSION
The study sought to define the urine bacteriology of patients with symptomatic BPH in our region. Bladder outlet obstruction due to BPH is a major cause of bacteriuria in aging males. This is as a result of increased bacterial colonization of the urinary tract due to stasis and incomplete bladder emptying. Urethral catheterization and cystoscopic evaluation also increase the risk of bacteriuria in these patients.[4]
The study noted bacteriuria in 44.7% of the patients. The observed rate is higher than 4.4%, 15%, and 20.6% noted in previous studies in Japan, Saudi Arabia, and Spain, respectively.[6,7,10] The reason for this difference is not very obvious. This finding is worrisome as patients with indwelling catheters, an established risk factor for bacteriuria, included in the previous studies were excluded from ours. The higher incidence of bacteriuria noted may be related to the long mean duration of lower urinary tract symptoms in our patients.
The study did not reveal any significant difference between the mean ages of patients with and those without bacteriuria. This is in contrast with findings from previous studies, which noted a higher incidence of bacteriuria in the older males.[6,7,10] This finding from the above studies has been partly attributed to the decreasing concentration of prostatic zinc-associated antimicrobial factor and increased alkalinity of urine in elderly males.[5] In addition, there was no significant difference between the mean postvoid residual volumes (not recorded in those with acute retention) of patients with and those without bacteriuria. Although men with large postvoid residual volume are at a greater risk of bacteriuria, there is no consensus on the cut-off value that would predict positive urine culture with sufficient sensitivity and specificity.[11,12]
Most cases of bacteriuria are caused by organisms in the enteric flora which periodically gain access to the genitourinary tract as a result of the proximity to the anal opening. Upon peri-urethral colonization, uropathogens gain access to the bladder by ascent through the urethra.[13] Other cases of bacteriuria may be caused by cross-contamination from other patients and healthcare personnel or from contaminated hospital materials such as catheters, dressing solutions, beddings, and bed pans.[13]
E. coli noted in 47.6% of the culture positive specimens was the most frequently isolated species. This finding is in keeping with those of previous studies within the region and abroad.[6,7,14,15,16] Other organisms noted included Klebsiella, Proteus, Pseudomonas, and Enterobacter species. The predominant presence of coliform organisms in the processed urine samples suggests that contamination by endogenous bowel flora is a major factor leading to bacteriuria in these aging males. Bacteriuria was monobacterial in 85.7% of cases whereas two different species were isolated from 6 urine samples.
The antibiotic susceptibility testing revealed a high degree of resistance to the cephalosporins and fluoroquinolones. This may have been due to the fact that these antibiotics have been abused in the past as a result of self-medication and inappropriate administration in our region.[17] The incidence of E. coli resistance recorded for ciprofloxacin, a fluoroquinolone commonly prescribed for the empiric treatment of UTI, was 75%. Similarly, ciprofloxacin resistance among E. coli strains has been on the upward trend over the years in different regions of the world,[18,19] especially among the aging population.[19,20] This poses a challenge in the empirical management of patients with UTI.
Newer beta lactam antibiotics such as the carbapenems recorded greater activity against the isolated organisms. These agents are expensive and are not suitable for routine prophylactic use due to cost, need for parenteral administration, and risk of development of resistant pathogens. The isolated organisms were sensitive to nitrofurantoin in 85.7% of cases. This is a relatively cheap antibiotic, available in oral formulations with proven activity against most Gram-negative uropathogens.[21] This finding suggests that there may be a greater role for this agent in prophylaxis and treatment of UTI in our region. A previous study noted an increase in the usage of nitrofurantoin from 14% to 30% in the treatment of uncomplicated UTI within a 5-year period as a result of the outcome of antibiotic susceptibility testing.[22]
The patients with bacteriuria who had clinical and microbiological features of UTI such as dysuria and pyuria received therapeutic doses of antibiotics based on susceptibility testing. The patients with negative urine cultures who underwent prostatectomy received antibiotic prophylaxis with intravenous ceftriaxone for 24 h based on existing unit protocol.
The presence of bacteriuria in patients with BPH has been associated with an increased incidence of wound infection and UTI after prostatectomy.[8] However, factors which may play a contributory role include preoperative urethral catheterization, length of surgical procedure, use of bladder irrigation, and antibiotic administration.[7,23]
Though the outcome of bacteriuria in the patients who underwent surgery was not studied, previous studies[7,24,25] have shown a reduction in the incidence of bacteriuria after prostatectomy with antibiotic prophylaxis. Pourmand et al. noted a reduction of bacteriuria from 15% to 3.3% with antibiotic administration.[7] Another study noted a 62% reduction in the rate of bacteriuria after transurethral resection of the prostate with fleroxacin administration.[24]
CONCLUSION
Bacteriuria is a common finding in patients with symptomatic BPH in our setting. Bacterial isolates were mostly sensitive to newer and more expensive carbapenems, but showed greater resistance to the cephalosporins and fluoroquinolones. Due to its relatively low cost and acceptable susceptibility profile, there may be a greater role for nitrofurantoin in the prophylaxis and treatment of complicated UTI in patients with BPH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bock-Oruma AA, Dienye PO, Oghu IS. Prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care. Port – Harcourt, Nigeria. S Afr Fam Med. 2013;55:467–72. [Google Scholar]
- 2.Amu OC, Udeh EI, Ugochukwu AI, Dakum NK, Ramyil VM. The value of international prostate symptom scoring system in the management of BPH in Jos, Nigeria. Niger J Clin Pract. 2013;16:273–8. doi: 10.4103/1119-3077.113446. [DOI] [PubMed] [Google Scholar]
- 3.Nekic VC, Tiljak H, Petricek G, Soldo D, Nekic G, Buljan N. Quality of life assessment of the male with benign prostate hypertrophy. Acta Med Croatica. 2007;61:49–55. [PubMed] [Google Scholar]
- 4.Shortliffe LM, McCue JD. Urinary tract infection at the age extremes: Pediatrics and geriatrics. Am J Med. 2002;113(Suppl 1A):55S–66S. doi: 10.1016/s0002-9343(02)01060-4. [DOI] [PubMed] [Google Scholar]
- 5.Gómez Y, Arocha F, Espinoza F, Fernández D, Vásquez A, Granadillo V. Zinc levels in prostatic fluid of patients with prostate pathologies. Invest Clin. 2007;48:287–94. [PubMed] [Google Scholar]
- 6.Fujita K, Murayama K, Ida T, Sumiyoshi Y, Yoshida K, Takaha M, et al. A cooperative study on the incidence of bacteriuria in patients with benign prostatic hypertrophy. Nihon Hinyokika Gakkai Zasshi. 1994;85:1348–52. doi: 10.5980/jpnjurol1989.85.1348. [DOI] [PubMed] [Google Scholar]
- 7.Pourmand G, Abedi AR, Karami AA, Khashayar P, Mehrsai AR. Urinary infection before and after prostatectomy. Saudi J Kidney Dis Transpl. 2010;21:290–4. [PubMed] [Google Scholar]
- 8.Richter S, Lang R, Zur F, Nissenkorn I. Infected urine as a risk factor for postprostatectomy wound infection. Infect Control Hosp Epidemiol. 1991;12:147–9. doi: 10.1086/646307. [DOI] [PubMed] [Google Scholar]
- 9.CLSI. CLSI Document M100-S21. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. Performance Standards for Antimicrobial Susceptibility Testing; Twenty First Informational Supplement. [Google Scholar]
- 10.Soler Soler JL, Hidalgo Domínguez MR, Martínez Torres JL, Zuluaga Gómez A, Nogueras Ocaña M, Lardelli Claret P, et al. Relationship between preoperative urine cultures and prostatic gland cultures in patients treated for benign prostatic hyperplasia. Actas Urol Esp. 1999;23:505–17. [PubMed] [Google Scholar]
- 11.Kaplan SA, Wein AJ, Staskin DR, Roehrborn CG, Steers WD. Urinary retention and post-void residual urine in men: Separating truth from tradition. J Urol. 2008;180:47–54. doi: 10.1016/j.juro.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 12.May M, Brookman-Amissah S, Hoschke B, Gilfrich C, Braun KP, Kendel F. Post-void residual urine as a predictor of urinary tract infection – Is there a cutoff value in asymptomatic men? J Urol. 2009;181:2540–4. doi: 10.1016/j.juro.2009.01.103. [DOI] [PubMed] [Google Scholar]
- 13.Najar MS, Saldanha CL, Banday KA. Approach to urinary tract infections. Indian J Nephrol. 2009;19:129–39. doi: 10.4103/0971-4065.59333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibadin MO. Childhood urinary tract infection in Benin City: Pathogens and antimicrobial sensitivity pattern. J Med Biomed Res. 2002;1:22–8. [Google Scholar]
- 15.Omoregie R, Igbarumah IO, Egbe CA, Ogefere H. Urinary tract infections among the elderly in Benin City, Nigeria. Fooyin J Health Sci. 2010;2:90–3. [Google Scholar]
- 16.Oladeinde BH, Omoregie R, Olley M, Anunibe JA. Urinary tract infection in a rural community of Nigeria. N Am J Med Sci. 2011;3:75–7. doi: 10.4297/najms.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vellinga A, Tansey S, Hanahoe B, Bennett K, Murphy AW, Cormican M. Trimethoprim and ciprofloxacin resistance and prescribing in urinary tract infection associated with Escherichia coli: A multilevel model. J Antimicrob Chemother. 2012;67:2523–30. doi: 10.1093/jac/dks222. [DOI] [PubMed] [Google Scholar]
- 19.Arslan H, Azap OK, Ergönül O, Timurkaynak F. Urinary Tract Infection Study Group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56:914–8. doi: 10.1093/jac/dki344. [DOI] [PubMed] [Google Scholar]
- 20.Karlowsky JA, Thornsberry C, Peterson DE, Mayfield DC, Sahm DF. Antimicrobial resistance among Escherichia coli urinary tract isolates in the United States: A current view provided by electronic surveillance. Infect Dis Clin Pract. 2001;10:87–92. [Google Scholar]
- 21.Garau J. Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: Fosfomycin, nitrofurantoin and tigecycline. Clin Microbiol Infect. 2008;14(Suppl 1):198–202. doi: 10.1111/j.1469-0691.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2002;162:41–7. doi: 10.1001/archinte.162.1.41. [DOI] [PubMed] [Google Scholar]
- 23.Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP) – Incidence, management, and prevention. Eur Urol. 2006;50:969–79. doi: 10.1016/j.eururo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 24.Viitanen J, Malminiemi K, Kallio J, Puolakka VM, Rajala P, Tammela TL. Transurethral prostatectomy in patients with preoperative bacteriuria: Double-blind study with oral fleroxacin and cefalexin. Chemotherapy. 1998;44:69–75. doi: 10.1159/000007093. [DOI] [PubMed] [Google Scholar]
- 25.Berry A, Barratt A. Prophylactic antibiotic use in transurethral prostatic resection: A meta-analysis. J Urol. 2002;167(2 Pt 1):571–7. doi: 10.1016/S0022-5347(01)69088-8. [DOI] [PubMed] [Google Scholar]


