Abstract
We previously reported that bone marrow grafts from matched sibling donors resulted in best graft-versus-host disease-free, relapse-free survival at 1-year post allogeneic hematopoietic cell transplantation. However, pediatric patients comprised the majority of bone marrow graft recipients in that study. To better define this outcome in adults and pediatric patients at 1- and 2-years post- allogeneic hematopoietic cell transplantation, we pooled data from the University of Minnesota and the Hôpital Saint-Louis in Paris, France (n=1901). Graft-versus-host disease-free, relapse-free survival was defined as the absence of grade III–IV acute graft-versus-host disease, chronic graft-versus-host disease (requiring systemic therapy or extensive stage), relapse and death. In adults, bone marrow from matched sibling donors (n=123) had best graft-versus-host disease-free, relapse-free survival at 1- and 2-years, compared with peripheral blood stem cell from matched sibling donors (n=540) or other graft/donor types. In multivariate analysis, peripheral blood stem cells from matched sibling donors resulted in a 50% increased risk of events contributing to graft-versus-host disease-free, relapse-free survival at 1- and 2-years than bone marrow from matched sibling donors. With limited numbers of peripheral blood stem cell grafts in pediatric patients (n=12), graft-versus-host disease-free, relapse-free survival did not differ between bone marrow and peripheral blood stem cell graft from any donor. While not all patients have a matched sibling donor, graft-versus-host disease-free, relapse-free survival may be improved by the preferential use of bone marrow for adults with malignant diseases. Alternatively, novel graft-versus-host disease prophylaxis regimens are needed to substantially impact graft-versus-host disease-free, relapse-free survival with the use of peripheral blood stem cell.
Introduction
Disease relapse and graft-versus-host disease (GVHD) impact the length and quality of life after allogeneic hematopoietic cell transplantation (HCT). Yet, in clinical trials, outcomes of allogeneic HCT are often defined by isolated events of GVHD, relapse, and mortality. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) incorporated these major causes of morbidity and mortality into a composite endpoint named GVHD-free, relapse-free survival (GRFS) - defined as the absence of grade III–IV acute GVHD, chronic GVHD requiring systemic immunosuppression, relapse, or death. We previously reported 1-year GRFS of 31% in 907 consecutive patients who underwent allogeneic HCT at the University of Minnesota (UMN) from 2000–2012.1 In that study, bone marrow (BM) grafts from a human leucocyte antigen (HLA)-matched sibling donor (MSD) resulted in the best GRFS (51%) as compared with peripheral blood stem cell (PBSC) from MSD (25%), BM from matched unrelated donors (URD) (32%), or umbilical cord blood (UCB) transplants (31%). However, there were limited numbers of adult patients (age >21) who received BM from MSD, and thus specific conclusions regarding the impact of graft source in adults could not be drawn. To address that question in a larger dataset, we now report the outcomes of 1,901 patients pooled from the UMN and the Hôpital Saint-Louis in Paris (Saint-Louis). Moreover, in our previous study, pediatric recipients of BM from MSD served as the reference group to which all other groups (including adult patients) were compared. In the current study, we report GRFS at 1- and 2-years post- transplantation in pediatric and adult patients independently, as their GRFS differs widely. With our pooled data, we show that allogeneic HCT with BM from a MSD results in the best GRFS in adult patients at both 1- and 2-years. In contrast, pediatric patients have similar GRFS with PBSC or BM graft from any donor, but the use of UCB was associated with inferior GRFS.
Methods
Our primary objective was to compare GRFS among different donor/graft sources at 1- and 2-years. The secondary objectives were (a) to define the distribution of GRFS events at 1-year among different donor/graft sources and (b) to define disease free survival (DFS) and overall survival (OS) at 1- and 2-years post-HCT.
Patient population and definitions
We included all consecutive patients who underwent first allogeneic HCT for hematological malignancy at the UMN from 2000 to 2013 (n=995) or Saint-Louis from 2000 to 2012 (n=906). Graft sources included PBSC, BM and UCB, while donors were MSD or matched URD (6/6-HLA matched), other related donors (5-6/6-HLA matched), or mismatched URD (5/6-HLA matched). Patients with prior allogeneic HCT, recipients of syngeneic HCT, haploidentical HCT, experimental cellular therapies or graft manipulation techniques, and those with non-malignant diseases were excluded. GRFS events were defined at 1- and 2- years of HCT as the first occurrence of grade III–IV acute GVHD, extensive or systemic chronic GVHD requiring therapy, relapse, or death. Disease risk at the time of transplantation was classified into standard-risk or high-risk based on the American Society for Blood and Marrow Transplantation (ASBMT) 2006 risk scoring schema.2 Acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in first or second complete remission, chronic myeloid leukemia (CML) in first chronic phase, Hodgkin or non-Hodgkin lymphoma in complete remission or chemotherapy-sensitive partial remission, chronic lymphocytic leukemia (CLL) in first remission were defined as standard-risk; all other diseases were classified as high-risk. DFS was defined as the time from transplantation to relapse of the underlying malignancy or death, and OS was defined as the time from transplantation to death. All HCT and data collection protocols were reviewed and approved by the University of Minnesota Institutional Review Board.
Statistical methods
Data were analyzed independently for pediatric patients (age <21 years) and adults (age>21 years). The Wilcoxon signed-rank test was used to compare characteristics across centers for continuous factors and the Chi-square test was used for categorical variables. Kaplan-Meier curves were used to estimate the probability of GFRS at 1- and 2-years post-HCT.3 The log-rank test was used to complete the comparisons. Direct adjusted survival curves were also calculated based on a stratified Cox model.4 The Cox regression model was used to examine the independent effect of factors on GFRS.5 Proportional hazards were checked using martingale residuals.6 Transplant centers violated the proportional hazards assumption, and therefore models were stratified by center. Other factors which were examined included year of HCT (2000–2007 versus 2008–2013, based on natural cut-off point), age, gender (male versus female), diagnosis, recipient cytomegalovirus (CMV) serostatus, type of conditioning (myeloablative versus reduced intensity conditioning (RIC), with or without anti-thymocyte globulin (ATG)), GVHD prophylaxis, donor type (MSD versus other related donor versus matched URD versus mismatched URD versus UCB), graft type (BM versus PBSC versus UCB) and disease risk (standard versus high-risk). All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). All reported P-values are 2-sided.
Results
Patient and treatment characteristics
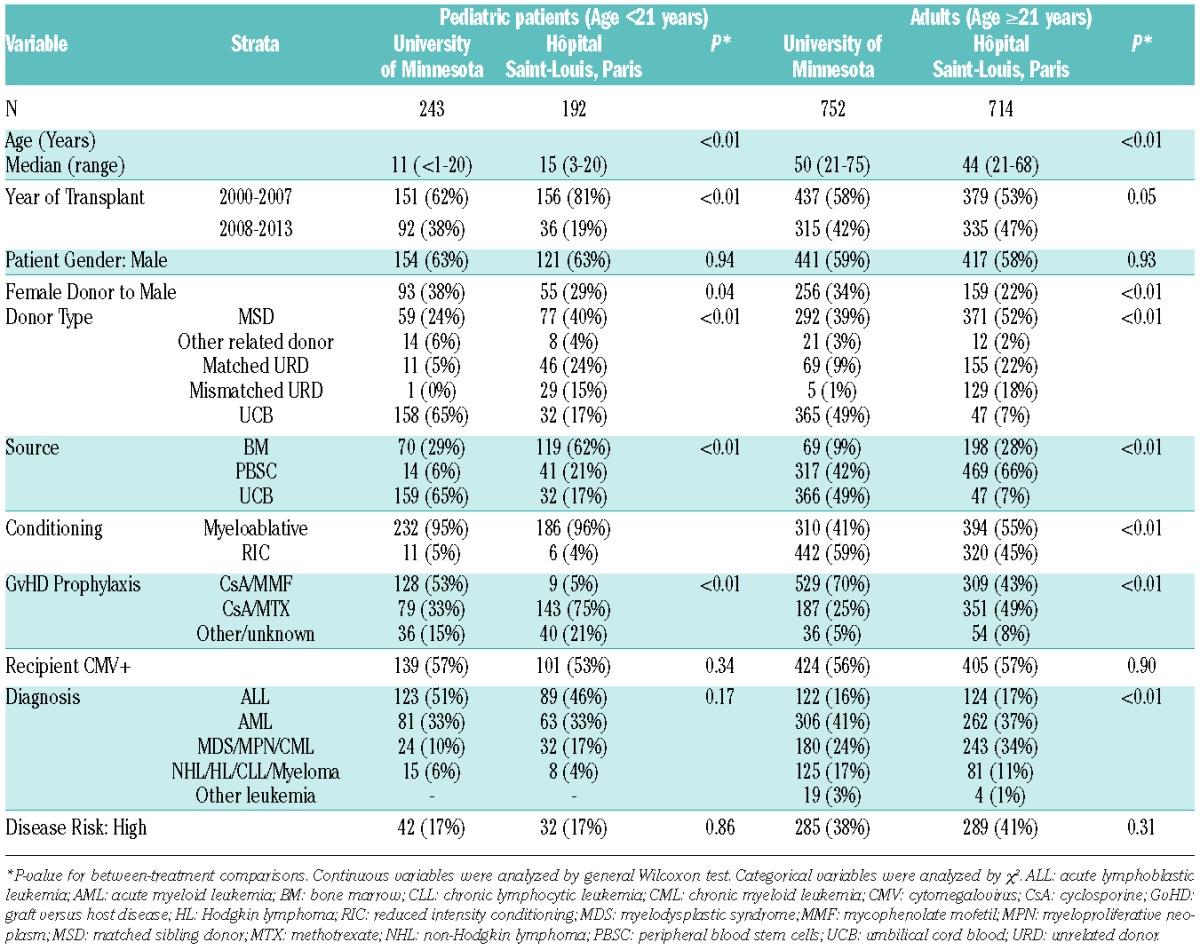
A total of 1901 patients, including 1466 adults were analyzed, of which 456 received BM grafts (Table 1). Overall, sixty percent of patients were males, 56% were CMV seropositive, 61% had acute leukemia and 63% had standard-risk disease. As expected, there were significant differences in patient and treatment characteristics between the centers. More than half of the patients at the UMN received UCB as the graft source (53%), while PBSC was the most frequent graft source (56%) at Saint-Louis. Correspondingly, the most common donor type at Saint-Louis was MSD (49%), which constituted 35% of donor types at the UMN. Myeloablative conditioning regimens were used in a majority of patients at both the institutions, although more commonly at Saint-Louis (64%) than at the UMN (55%). Correlating with the differences in donor/graft preferences, the choice of GVHD prophylaxis also differed between the centers. Two-thirds of patients at the UMN received mycophenolate mofetil with cyclosporine for GVHD prophylaxis, while methotrexate and cyclosporine (55%) was used most commonly at Saint-Louis. The median overall follow-up was 6.2 years (range, 0.3–14.4 years) and was similar in both sites.
Table 1.
Baseline patient and treatment characteristics.
Estimates of GRFS, DFS and OS in adults at one- and two-years post-transplantation
The unadjusted Kaplan-Meier estimate of GRFS was 34% (95% confidence interval (C.I.) 31–36%) at 1-year and 27% (95% C.I. 25–29%) at 2-years. The estimates of DFS were 54% (95% C.I. 51–57%) and 46% (95% C.I. 43–49%) at 1-and 2-years, respectively, while those of OS were 62% (95% C.I. 60–65%) and 53% (95% C.I. 51–56%) at 1-and 2-years, respectively (Figure 1A; Online Supplementary Table S1A and S2A).
Figure 1.
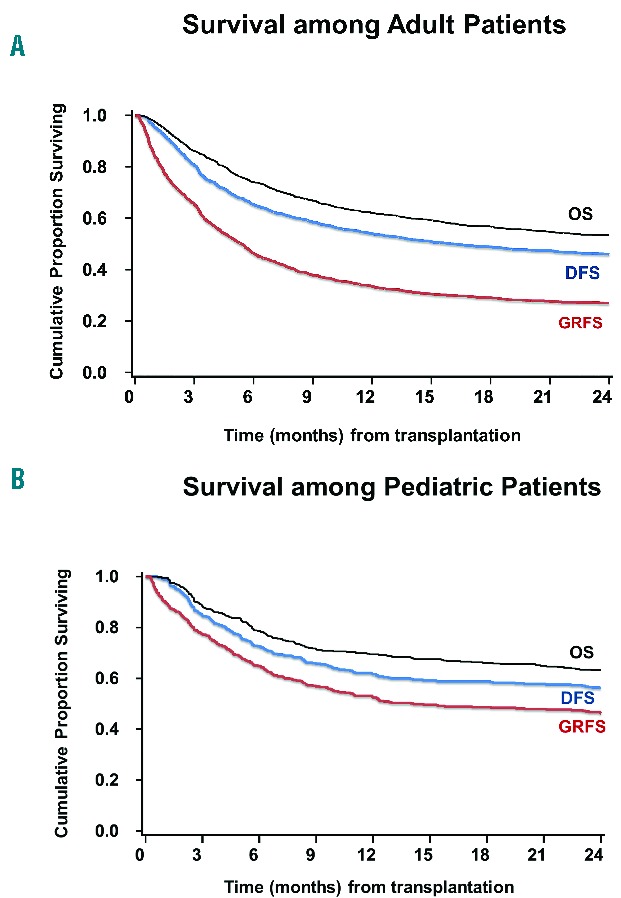
Kaplan-Meier estimates of GRFS, DFS and OS among (A) adults and (B) pediatric patients.
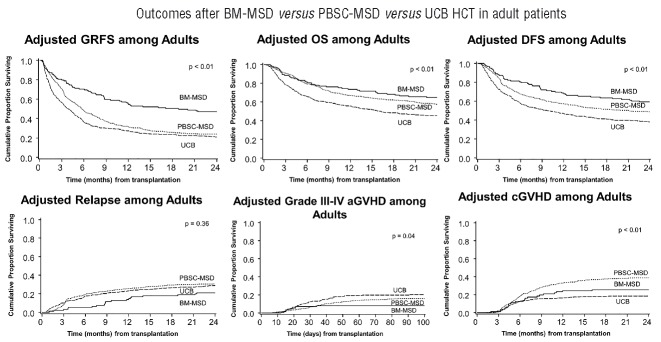
In univariate analysis, BM from MSD resulted in best GRFS at both 1-year (57%, 95% C.I. 48–65%) and at 2-years (49%, 95% C.I. 40–57%) compared with any other donor/graft source, P<0.001 (Table 2A). After adjusting for age, conditioning regimens, diagnosis, disease risk and recipient CMV serostatus in multiple regression analysis stratified by center, donor/graft source was found to be an independent predictor of GRFS (Table 3A). Figure 2 shows adjusted survival and adjusted GRFS-defining events in adults with BM versus PBSC graft from MSD versus UCB grafts. The use of PBSC from either MSD, matched URD or mismatched URD, and the use of UCB grafts resulted in a 50–120% increased risk of GRFS events at both 1- and 2-years compared with BM from MSD. However, BM from matched URD was associated with similar GRFS at 1- and 2-years as BM from MSD. The use of RIC was associated with a 20–30% lower incidence of GRFS events at 1- and 2-years compared with myeloablative regimens. The use of ATG with myeloablative regimens did not impact GRFS. Similarly, GRFS was not affected by age, after adjusting for other variables. Patients with high-risk disease had a 30% higher risk of GRFS events at 1- and 2-years compared to those with standard-risk disease. Recipient CMV serostatus did not impact GRFS.
Table 2A.
Univariate estimates of GRFS in adults (Age≥21 years).
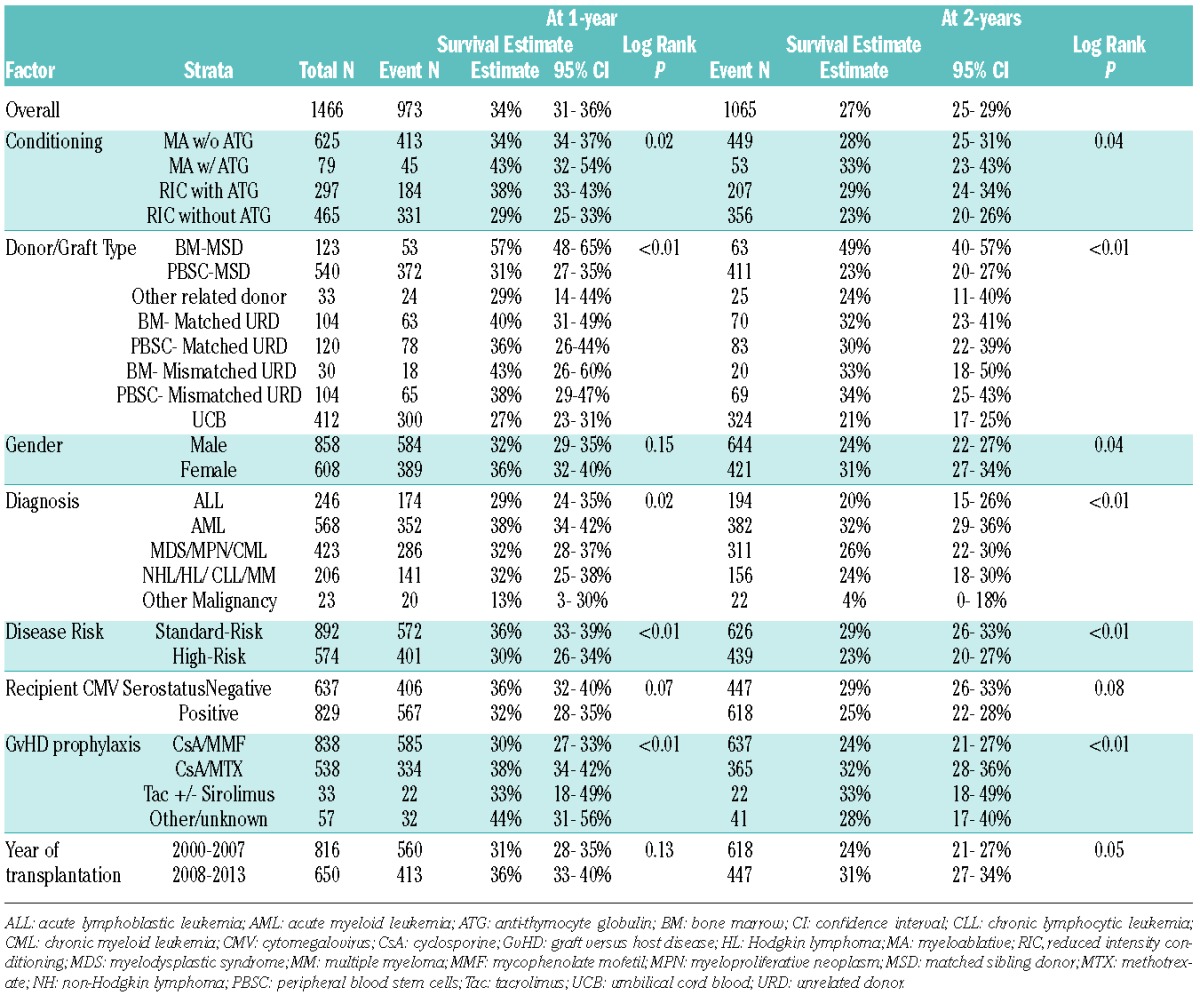
Table 3A.
Multiple regression analysis on risk of GRFS *(adult patients).
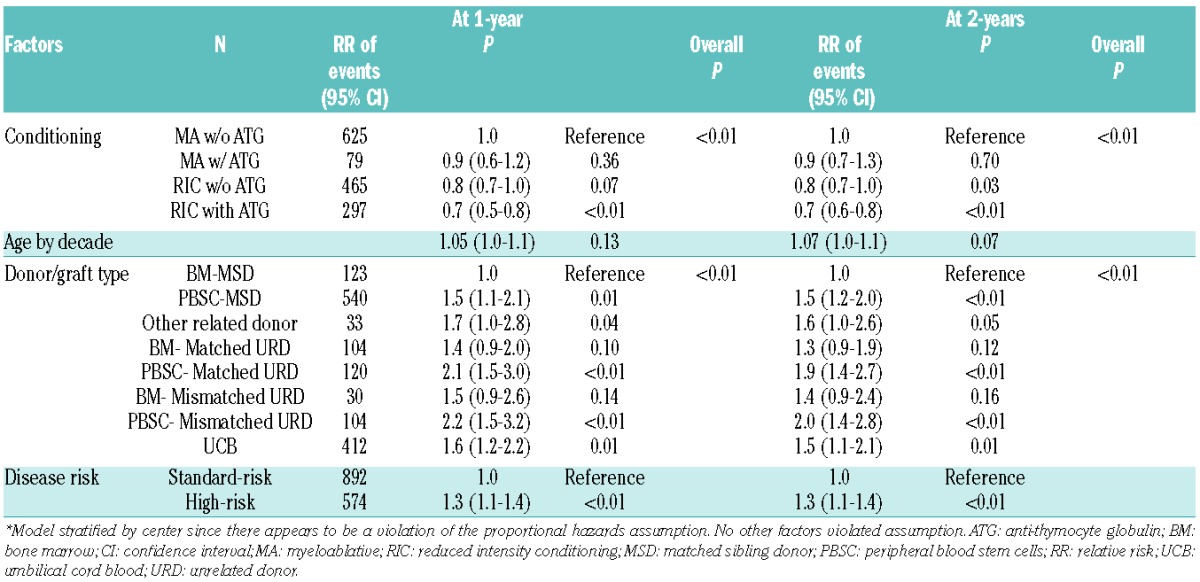
Figure 2.
Adjusted GRFS, DFS, OS, grade III–IV acute GVHD, chronic GVHD and relapse among adults with BM graft from matched sibling donor versus PBSC from matched sibling donor versus UCB graft.
Distribution of GRFS events in adults at one-year post-transplantation
The distribution of GRFS events at 1-year varied significantly among different graft types and donor sources, P<0.01. In the setting of MSD transplantation, chronic GVHD was the most frequent GRFS event in recipients of either PBSC (35%) or BM (30%) grafts. In contrast, acute GVHD was the most common GRFS event in recipients of PBSC (49%) or BM (33%) from matched URD and PBSC (49%) from mismatched URD. Acute and chronic GVHD were common (28% each) in recipients of BM from mismatched URD. Death (29%) and relapse (27%) contributed to a majority of GRFS events after UCB transplantation while GVHD events were less frequent. Events occurring after the GRFS-defining events also varied by donor/graft source (Online Supplementary Figures S1 and S2). Table 4A depicts one-year cumulative incidences of grade III–IV acute GVHD, chronic GVHD, relapse and death by donor/graft sources.
Table 4A.
Cumulative incidence/Kaplan-Meier estimate of each type of event (age ≥21 years).
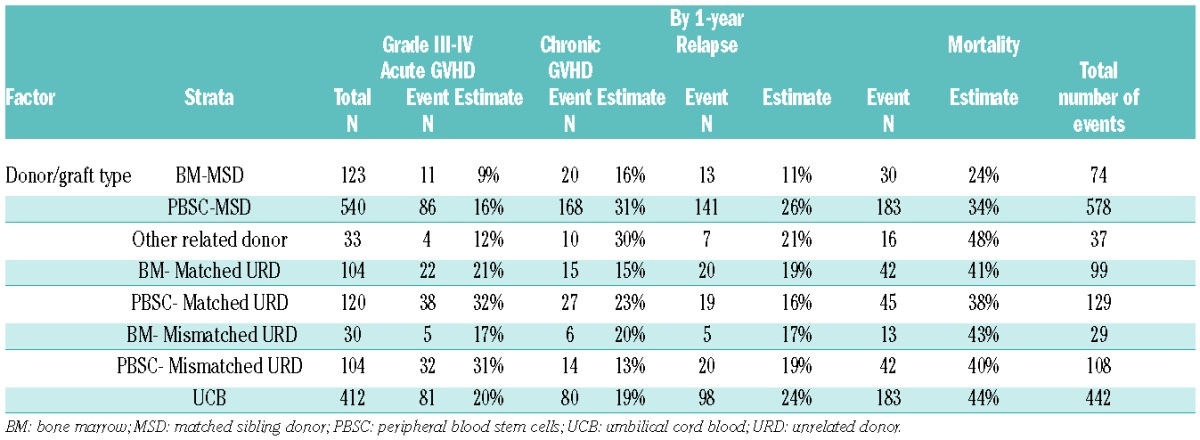
Analyzing by conditioning regimens, deaths were more common in recipients of myeloablative compared with RIC regimens (23% versus 16%), while relapse (21% versus 34%) accounted for more GRFS events in those who received RIC regimens. Major causes of death were infections (30%) in recipients of BM from MSD, disease relapse (46%) in PBSC from MSD, acute GVHD in either PBSC (53%) or BM (31%) from matched URD, and disease relapse (33%) and infection (22%) in UCB recipients (Online Supplementary Figure S3A).
Estimates of GRFS, DFS and OS in pediatric patients at one- and two-years post-transplantation
Pediatric patients had considerably superior estimated GRFS at 1-year (52%, 95% C.I. 48–57%) and at 2-years (46%, 95% C.I. 41–51%) compared with adults. Similarly, the estimates of DFS at 1-year (62%, 95% C.I. 57- 66%) and 2-years (56%, 95% C.I. 51- 61%) and OS at 1-year (69%, 95% C.I. 65- 74%) and at 2-years (63%, 95% C.I. 58- 68%) were higher than those in adults (Figure 1B; Online Supplementary Table S1B and S2B).
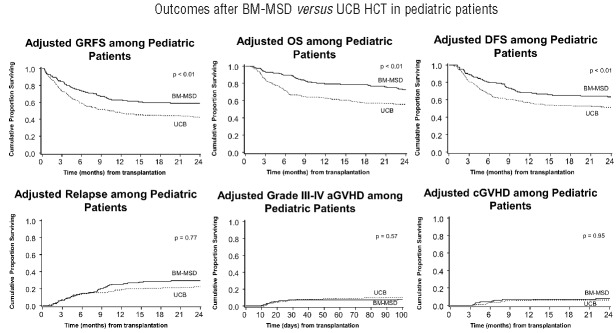
It is noteworthy that only 12 patients received PBSC from MSD contributing to limited GRFS events compared with 124 BM recipients from MSD. Within this limitation, GRFS differed significantly among various donor/graft sources in univariate analysis (Table 2B). However, in multiple regression analysis stratified by center, graft/donor source was not a statistically significant predictor of GRFS. All donor/graft sources had a similar risk of GRFS events at 1- or 2-years (overall P=0.17 and 0.15, respectively), excluding UCB, which was associated with 50–70% increased risk of GRFS events (Table 3B). Figure 3 shows the adjusted GRFS, DFS, OS and GRFS-defining events among recipients of BM graft from MSD compared with UCB graft –the two most common graft sources used in pediatric patients. The use of RIC resulted in 2.5–2.8 fold higher risk of GRFS events at 1- and 2-years compared to myeloablative conditioning. Disease risk and recipient CMV seropositivity were not associated with GRFS.
Table 2B.
Univariate estimates of GRFS in pediatric patients (Age <21 years).
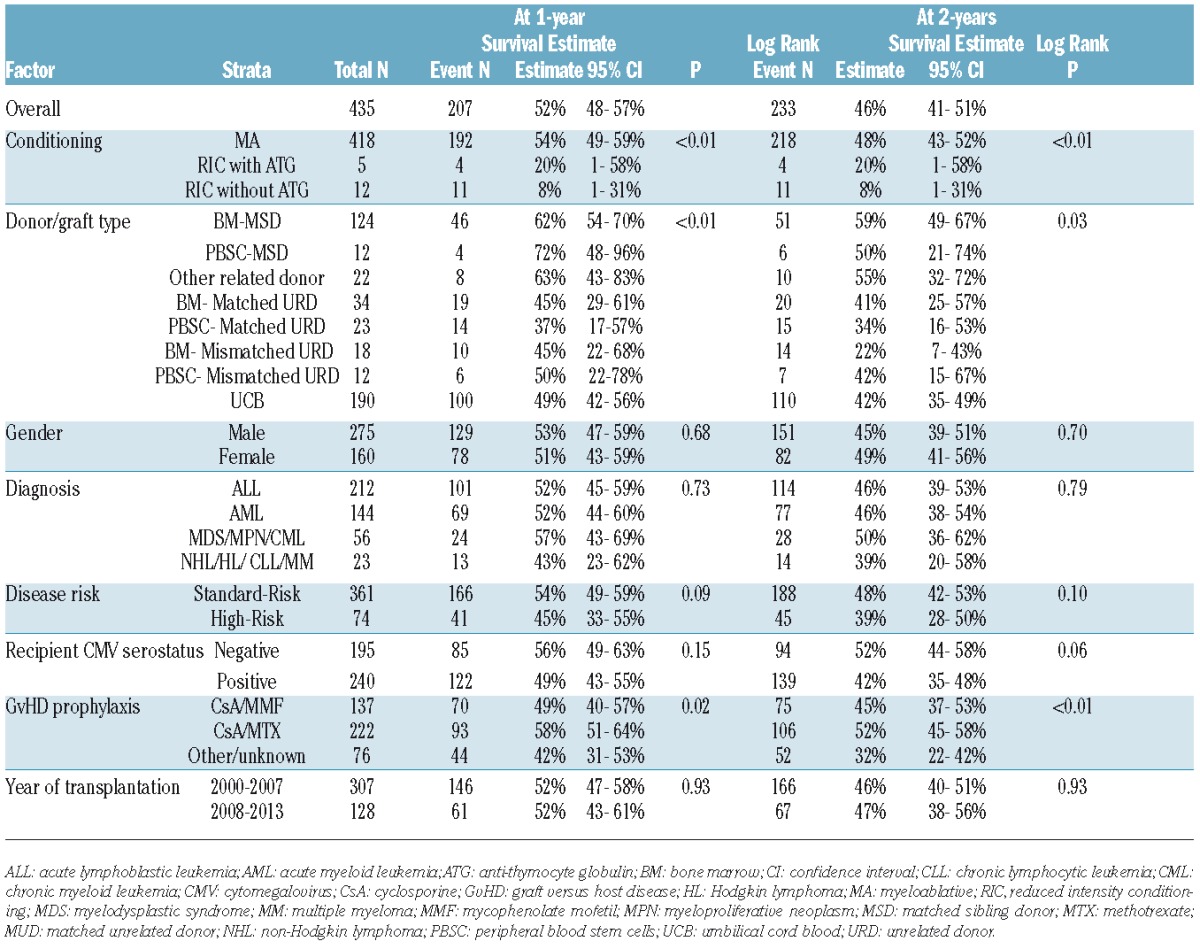
Table 3B.
Multiple regression analysis on risk of GRFS * (Pediatric patients).
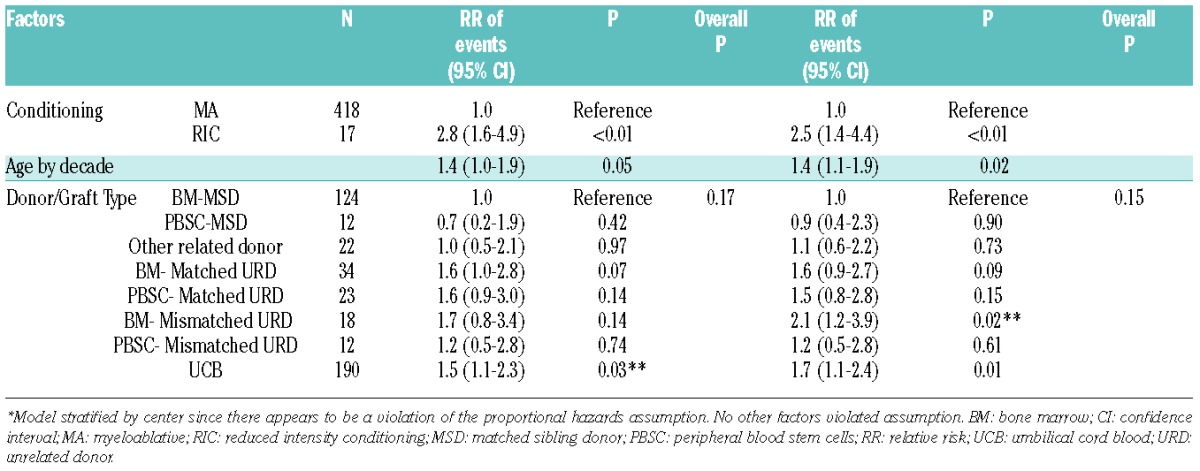
Figure 3.
Adjusted GRFS, DFS, OS, grade III–IV acute GVHD, chronic GVHD and relapse among pediatric patients with BM graft from matched sibling donor compared with UCB graft.
Distribution of GRFS events in pediatric patients at one -year post-transplantation
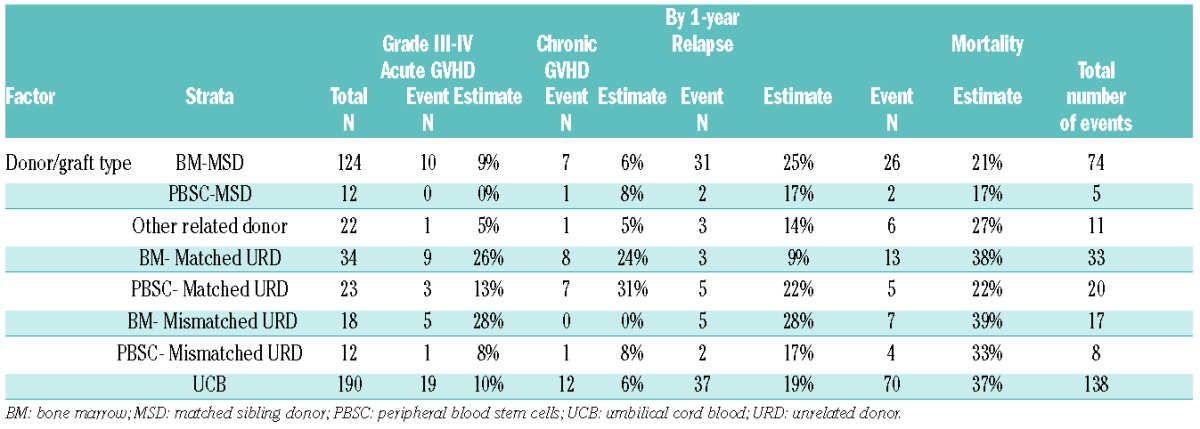
GRFS events differed across various graft/donor types, P<0.01. Relapse was the most frequent GRFS event in recipients of BM (59%) or PBSC (50%) from MSD. In the setting of matched URD transplantation, chronic GVHD (43%) in PBSC recipients and acute GVHD (47%) in BM recipients accounted for a majority of events. Recipients of BM from mismatched URD had either relapse (50%) or acute GVHD (50%) as the GRFS-defining event. Relapse (33% and 37%) and death (33% and 36%) added to more than two-thirds of GRFS events in recipients of PBSC from mismatched URD and UCB recipients, respectively. Online Supplementary Figures S1 and S2 depict the distribution of events occurring subsequent to GRFS-defining events by donor/graft source. In recipients of either myeloablative or RIC regimens, relapse (39% and 44%) was the most frequent event while chronic GVHD (14% and 3%) was the least frequent event contributing to GRFS; acute GVHD (23% and 25%) and death (24% and 19%) accounted for the rest. Disease relapse accounted for a vast majority of deaths in recipients of BM (69%) or PBSC (50%) from MSD (Online Supplementary Figure S3B). Cumulative incidences of grade III–IV acute GVHD, chronic GVHD, relapse and death at 1-year are shown in Table 4B.
Table 4B.
Cumulative incidence/Kaplan-Meier estimate of each type of event (age ≥21 years).
Discussion
In this study, we show that the use of BM from a MSD resulted in the best GRFS at 1-year (57%) and 2-years (49%) compared with other donor types and graft sources in adults. In multivariate analysis, BM from matched URD or even mismatched URD led to similar GRFS as BM from MSD, suggesting that BM may be the optimal graft source from adult HLA-matched related or unrelated donors in terms of GRFS. The use of UCB and PBSC from any donor were associated with 1.5–2 times higher risk of GRFS events than BM from MSD.
These findings are of interest, especially in view of the reported results of the randomized controlled trial in adult patients receiving PBSC or BM from matched URD, conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0201).7 That trial showed significantly higher incidence of chronic GVHD at 2-years in recipients of PBSC (53% versus 41%) and a higher incidence of graft failure in BM recipients (9% versus 3%), without any differences in acute GVHD, disease-free survival, or overall survival with BM versus PBSC.7 Despite the outcomes of BMT CTN 0201, PBSC continues to be the most frequently utilized graft source - representing about 65% of all grafts used for allogeneic HCT in recent years.8 In our study, both acute and chronic GVHD contributed to higher GRFS events in recipients of PBSC (49% and 22%) compared with BM (33% and 14%) from matched URD. Moreover, deaths due to GVHD were higher in PBSC (53%) than in BM (31%) recipients. Graft failures did not contribute to any deaths in recipients of either BM or PBSC from matched URD. Similar findings were noted in the MSD group where the proportion of acute or chronic GVHD events and deaths associated with them were higher in PBSC recipients compared with BM recipients. In addition to donor/graft source, other factors that had a favorable impact on GRFS included RIC regimen with ATG and standard risk disease in adults. Although these factors and the availability of a donor cannot be modified, GRFS could be favorably influenced by the preferential use of BM grafts rather than PBSC. However, despite these data, transplant centers and donors may continue the preferential use of PBSC for many reasons.9–11 Novel GVHD prophylaxis regimens are therefore currently undergoing evaluation for their impact on GRFS after PSBC transplantation.12
On the other hand, in the pediatric population, existing data distinctly show that the use of PBSC from MSD is associated with a higher risk of chronic GVHD, treatment related mortality, treatment failure and mortality compared with BM grafts.13 Given these data, only 12 patients in our study received PBSC from MSD compared with 124 BM grafts from MSD. Consequently, within the limitations of the sample size, GRFS did not differ between different donor or graft sources in pediatric patients, although UCB was associated with inferior GRFS. The only factor that favorably affected GRFS was the use of myeloablative conditioning compared with the RIC regimen. However, patients who received RIC regimens had comorbidities or contraindications to receive myeloablative regimens, such as prior use of high dose radiation therapy, recent autologous HCT, suspected or proven fungal pneumonia, prior CNS malignancy or veno-occlusive disease. Two patients received sequential intensive chemotherapy and RIC HCT for high risk AML.
It is important to recognize that GRFS is computed by a time-to-event analysis, which disregards all incidents happening after the onset of the first GRFS-defining event. Therefore, patients who develop GRFS-defining GVHD or relapse but completely recover with treatment may be morbidity-free at 1-year and are yet included among the GRFS events. To account for this shortcoming of GRFS analysis, real-time periodic assessments and analysis with specific survival models are needed. Another proposed composite outcome for GVHD treatment trials is failure-free survival,14–16 where the outcomes are measured from the time of onset of acute or chronic GVHD, which include the absence of second-line systemic therapy for GVHD, non-relapse mortality and recurrent malignancy during initial treatment of GVHD. Yet, it does not consider events happening prior to the onset of GVHD. Also, as mentioned above, BM and UCB constituted the majority of the graft sources in the pediatric population, because of which caution is warranted when comparing other donor/graft sources. In this study, we were unable to include hematopoietic cell transplantation comorbidity index (HCT-CI)17 or the revised disease risk index18 for risk stratification of all patients. Our study also does not address the importance of GRFS in “alternative donor” settings such as haploidentical HCT compared with UCB and mismatched HCT, which should be assessed in future studies.
It is critical to consider GRFS when deciding about a donor/graft source, in addition to independent outcomes of GVHD, disease-free survival and overall survival, because GRFS potentially represents a surrogate endpoint for quality-of-life. In our study, although 64% of all patients were alive and 56% were disease-free at 1-year, only 38% were free of any GRFS defining event at that time. Analysis of GRFS outcomes in the BMT CTN 0201 trial or in larger datasets, such as those available through the Center for International Blood and Marrow Transplant Research, will shed even more light on this crucial question. These data reignite the question of the optimal donor/graft source in adults, challenge the current practice standards, and emphasize the need for continued studies to address the key events leading to morbidity and mortality in HCT recipients given that a minority of patients still survive 1 year without a GRFS event.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/6/764
Funding
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B, B.R.B.).
References
- 1.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ASBMT. American Society for Blood and Marrow Transplantation RFI 2006; 2006. [Google Scholar]
- 3.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 4.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35(8):669–674. [DOI] [PubMed] [Google Scholar]
- 5.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 6.Collett D. Modelling Survival Data in Medical Research, Second Edition (Chapman & Hall/CRC Texts in Statistical Science) In: Chatfield C, ed; 2003. [Google Scholar]
- 7.Anasetti C, Logan BR, Confer DL, Blood, Marrow Transplant Clinical Trials N. Peripheral-blood versus bone marrow stem cells. N Engl J Med. 2013;368(3):288. [DOI] [PubMed] [Google Scholar]
- 8.Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. Available at: http://www.cibmtr.org; 2014.
- 9.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Lower risk for serious adverse events and no increased risk for cancer after PBSC vs BM donation. Blood. 2014;123(23):3655–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121(1):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young JH, Logan BR, Wu J, et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol Blood Marrow Transplant. 2016;22(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BMT CTN Protocol 1203 (PROGRESS I), available online at https://web.emmes.com/study/bmt2/protocol/1203_protocol/1203_protocol.html; 2015.
- 13.Eapen M, Horowitz MM, Klein JP, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol. 2004;22(24):4872–4880. [DOI] [PubMed] [Google Scholar]
- 14.Inamoto Y, Flowers ME, Sandmaier BM, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124(8):1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inamoto Y, Storer BE, Lee SJ, et al. Failure-free survival after second-line systemic treatment of chronic graft-versus-host disease. Blood. 2013;121(12):2340–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer J, Chai X, Martin PJ, et al. Failure-free survival in a prospective cohort of patients with chronic graft-versus-host disease. Haematologica. 2015;100(5):690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]