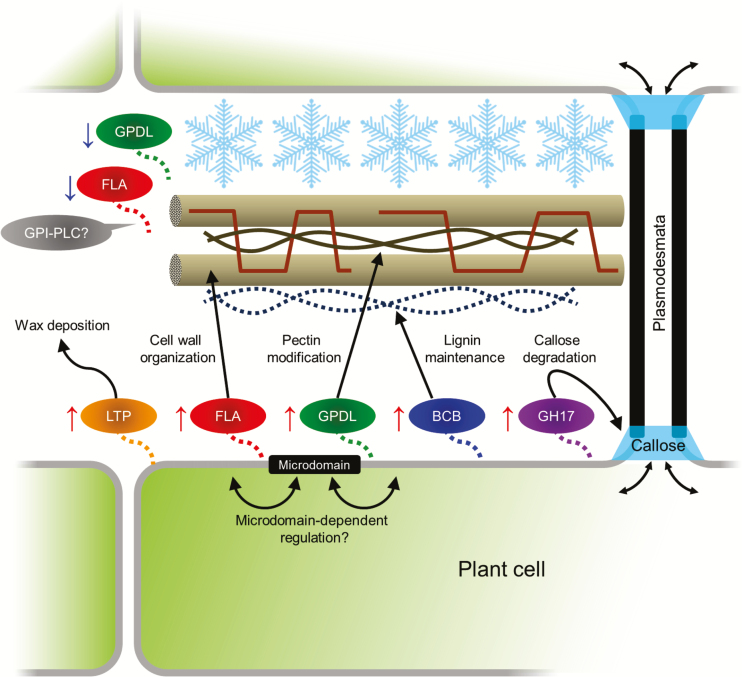
Fig. 7.
Schematic presentation of potential roles of representative GPI-APs identified in the present study in cell wall changes during cold acclimation. In the present study, 163 GPI-APs were identified in total. Among them, lipid transfer proteins (LTPs), fasciclin-like arabinogaractan proteins (FLAs), glycerophosphoryldiester phosphodiesterase-like proteins (GPDLs), blue-copper-binding protein (BCB), and O-glycosyl hydrolase family 17 proteins (GH17) predominantly increased during cold acclimation (CA). The proteins included in the four protein families have potential roles in wax deposition, cell wall organization, cellulose and pectin modification, and lignin synthesis and callose degradation, respectively. Although detailed mechanisms are not yet fully understood, physical and biochemical properties of the extracellular matrix including the cell wall are considered to be modified during cold acclimation in order to withstand extracellular ice propagation and freeze-induced dehydration. This study proposed the possibility that GPI-APs could be involved in remodeling of cell wall and plasmodesmal communication during cold acclimation, and PM microdomains may regulate cold-acclimation-associated activities of GPI-APs. On the other hand, changes of GPI-APs in the apoplast fraction were vastly different from those in the PM and GPI-AP/PI-PLC (+) fractions, suggesting that the activity and/or cellular localization is partly regulated by GPI-PLC.