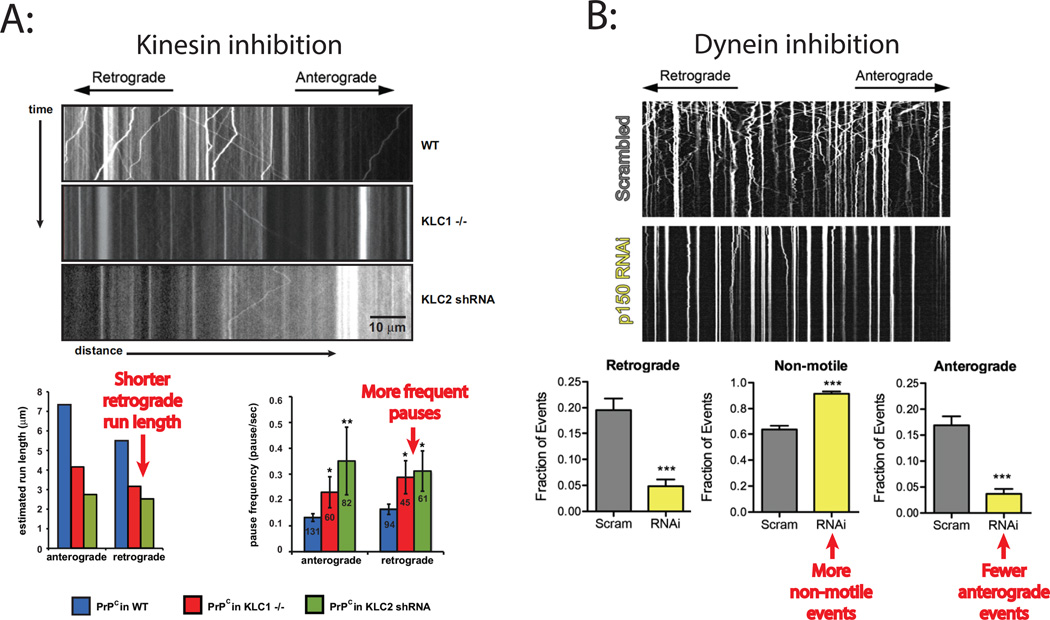
Figure 4. Examples of antagonistic motor codependence.
A: The anterograde motor kinesin was inhibited in mouse neurons by knocking out kinesin light-chain 1 (KLC1−/−) or by knocking down light-chain 2 (KLC2 shRNA), both of which are involved in linking kinesin-1 to PrPc vesicles. Vesicles were labeled by YFP-PrPc and their transport dynamics analyzed by kymographs. The wild-type kymograph (top) shows left and right diagonal tracks, indicative of retrograde and anterograde cargo transport, respectively, as well as some vertical lines indicative of stationary vesicles. In contrast, the kinesin inhibition kymographs (middle and bottom) show very little transport in either the retrograde or anterograde directions, and many stationary vesicles. Schematic plots of the data demonstrate that while kinesin inhibition was expected to result in longer retrograde run lengths and fewer pauses during retrograde movement, the opposite was observed. (While the observed results in the schematic approximate the published data, the expected results are only qualitative estimates for comparison). Figures adapted from Encalada et al.8. B: Dynein in mouse neurons was inhibited by knocking down the p150 subunit of the dynein adaptor protein dynactin that links dynein to vesicles. Vesicles were labeled by RFP-LAMP1 and their transport analyzed by kymographs. In the control kymograph at top (scrambled RNAi), numerous bidirectional transport events (diagonal lines) were observed, while following dynein inhibition (p150 RNAi) very little transport in either direction was observed and almost all vesicles were stationary (vertical lines). Schematic plots of the data show that while dynein inhibition is expected to result in more anterograde transport events and fewer non-motile events, the opposite was observed. Figure adapted from Moughamian and Holzbaur106.