Fig. 1.
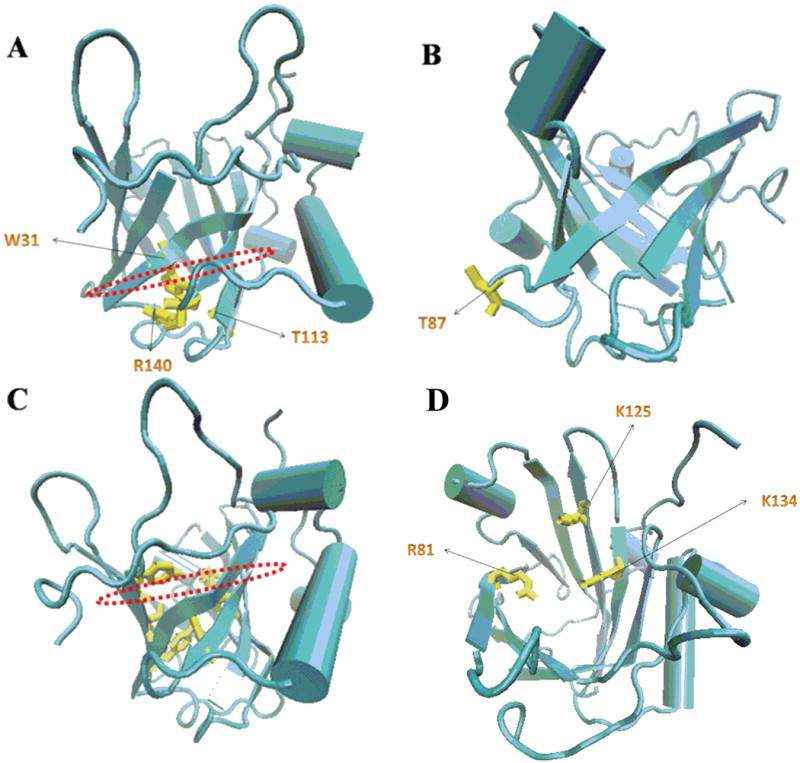
(A) The side chains cluster of the conserved residues W31, T113 and R140 (Yellow) together with the 310-helix closed the smaller end of the barrel at the bottom of the calyx. (B) The free SH group of residue C87 (Yellow) which associated with MMP-9 lied in an inter-strand loop at the closed end of the calyx. (C) The putative binding sites for lipophilic ligands were those hydrophobic aromatic and aliphatic residues including F27 (from the 310-helix), W31 and V33 (β1), V66 (β3), F83 (β4), F92 and L94 (β5), V108 and V110 (β6), and V121 and F123 (β7) (Yellow) at the base of the barrel. (D) Positively charged side-chains of two lysine residues (K125 and K134) and that of R81 were projected into the top open end of the barrel, which bind hydrophilic siderophores such as catecholates.
