Abstract
Background
Although several preclinical studies have shown that bone marrow cell (BMC) transplantation promotes cardiac recovery after myocardial infarction (MI), clinical trials with unfractionated bone marrow have shown variable improvements in cardiac function.
Methods
To determine whether in a population of post-MI patients, functional recovery after BM transplant is associated with specific BMC subpopulation, we examined the association between BMCs with left ventricular (LV) function in the LateTIME-CCTRN trial.
Results
In this population, we found that older individuals had higher numbers of BM CD133+ and CD3+ cells. BM from individuals with high body mass index had lower CD45dim/CD11bdim levels, while those with hypertension and higher C-reactive protein levels had higher numbers of CD133+ cells. Smoking was associated with higher levels of CD133+/CD34+/VEGFR2+ cells and lower levels of CD3+ cells. Adjusted multivariate analysis indicated that CD11bdim cells were negatively associated with changes in LV ejection fraction (LVEF) and wall motion in both the infarct and border zones. Change in LVEF was positively associated with CD133+, CD34+ and CD45+/CXCR4dim cells as well as faster BMC growth rates in endothelial colony forming assays.
Conclusions
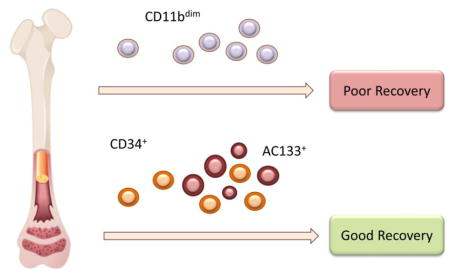
In the LateTIME population, BM composition varied with patient characteristics and treatment. Irrespective of cell therapy, recovery of LV function was greater in patients with greater BM abundance of CD133+ and CD34+ cells and worse in those with higher levels of CD11bdim cells. BM phenotype might predict clinical response prior to BMC therapy and administration of selected BM constituents could potentially improve outcomes of other future clinical trials.
Trial Registration
Clinical Trial Registration: clinicaltrials.gov Identifier NCT 00684060
Keywords: Bone marrow, heart failure, hypertension, stem cells, cell therapy
Graphical Abstract
Introduction
Although studies in animal models show that treatment of acute myocardial infarction (AMI) with bone marrow cells (BMCs) promotes tissue recovery 1,2, the clinical benefits of BMC therapy have been modest and inconsistent amongst studies. While some studies have shown improvements in cardiac function after BM transplant, others have failed to demonstrate significant benefits of cell therapy. 3–6. In the LateTIME-CCTRN trial, for example, there was no overall improvement in global or regional cardiac function, 6 months after intracoronary infusion of autologous BMC in the patient cohort as a whole 7. However because some individuals showed improvements while others did not it remained unclear whether improvements in left ventricular (LV) function in some individuals, but not others, may be related, at least in part, to differences in endogenous BMC phenotypes and patient demographics.. The BM is not a homogenous cell population. It contains a variety of stem and progenitor cells as well as hematopoietic cells that, in principle, could variably affect clinical outcomes. Because, BM characteristics vary as a function of age, treatment, disease, acute clinical events, and probably other as yet unrecognized factors 8, it is conceivable that the potency of autologous BM products varies depending upon the characteristics of the product aspirated from individual patients. Nevertheless, the effects of patient characteristics on BMC are unclear, and the influence of BM composition on outcome of BMC therapy remains unknown.
The goal of this study was to examine potential associations between patient demographics and BMC constituents in the LateTIME trial, and to determine whether specific BMC subpopulations and characteristics measured at baseline could predict improvements in cardiac function.
To determine whether variations in BMC composition due to different patient characteristics could affect outcomes, we examined the association between patient and cell characteristics at baseline. We then assessed the relationship between the cell characteristics and cardiac function. Our results show that cardiovascular disease (CVD) risk factors significantly affect the composition and characteristics of the BM, and that the levels of endothelial precursors and inflammatory cells within the BM are associated with myocardial recovery following cell therapy.
Methods
The LateTIME prospective cohort study comprised of patients consecutively enrolled in the clinical trial who participated in the 6-month follow-up and provided written consent to have their excess BMCs stored for further analyses at the CCTRN Biorepository. These analyses were pre-specified in the ancillary functional studies section of the CCTRN LateTIME protocol. The primary outcome comprised of changes in global (LVEF) and regional LV function (infarct zone (WMIZ) and border zone (WMBZ)) between baseline and 6 months as measured by cardiac magnetic resonance (CMR) imaging. As reported previously, the infarct zone was defined as the segments with the largest two signal intensity enhancement measures with gadolinium (using a 17 segment model). The border zone was defined as the region adjacent to the infarct zone in which the signal intensity enhancement was between 10% and 75%. Secondary endpoints included changes in CMR LV end systolic (LVESVI) and end diastolic volume indices (LVEDVI) and infarct size 7. To improve quantification of infarct size, the data were reanalyzed using a CMR algorithm to correct for LV mass 9. Cell populations in the BM were characterized by flow cytometry as previously described 9,10.
Functional Analysis
Trypan blue exclusion was used to assess viability of the cells that underwent functional analysis. BMC function was evaluated with 3 separate assays, such as (1) the colony-forming unit Hill (CFU-Hill), (2) the endothelial-colony forming cell (ECFC), and (3) the colony-forming unit fibroblast (CFU-F) assays.9 The number of colony forming units, type of colonies, and percent confluency were recorded on days 7, 14, 21, and 28 for the ECFC and CFU-F assays and on days 4 through 9 for the CFU-Hill assay. Results from all 3 assays were assessed with the following 3 metrics, such as (1) slope of the best-fit linear curve for the percent confluency over time, (2) exponential constant of the best-fit exponential curve for the number of colonies over time, and (3) maximum number of colonies present during the entire culture period.
Statistical Analysis
To examine the relationship between the frequency and growth characteristics of different BMC populations, the levels of different cell types in the BM were divided into two strata representing low (below median) and high (above median) levels. Because similar results were obtained with arithmetic and natural logarithms, the values of the parameters were not log-transformed. In the first set of analyses, we assessed the relationship between cell and patient characteristics, using standard regression analyses.
Composite Cardiac Impairment Score (CCIS)
To identify the specific BMC characteristics associated with cardiac functional outcomes, we analyzed whether baseline BMC characteristics of patients predict functional recovery. Given that there were over 40 phenotypes and six measures of LV function, a complete evaluation of the outcome relationships would be challenging and potentially redundant, therefore to examine whether there was high correlation between the variables (indicating that multiple variables may be measuring the same structure) we evaluated the correlation between: 1) different cell phenotypes and function; and 2) individual indices of cardiac performance. We conducted a principal components analysis (PCA) to take advantage of this correlation structure 11 and produce a composite cardiac function score. Principal component analysis (PCA) was used to: 1) identify and encapsulate what the original variables measured in common; and 2) are not correlated with one another 11,12. CCIS was calculated as:
The units of the constants are such that the resulting score is unit-less. The mean value of CCIS at baseline was 50.5 with a SD of 30.7. Its median value was 46.6 (21.1 to 133). The CCIS was higher when the values of LVEDVI, LVESVI, and infarct size were large and when the values of LVEF, WMIZ, and WMBZ were low. It explains 56.2% of the total variability in the system, i.e., one variable explains over half of the variability that the original 6 variables explained. The relationship between each of the traditional indices of LV function (LVEF, LVESVI, LVEDVI, WMIZ, WMBZ, and infarct size) and baseline cell characteristics was examined using regression analysis. In addition to these variables, ΔCCIS was computed from the change in CCIS from baseline to 6 months follow-up. The values of ΔCCIS were further characterized as a dichotomous variable, and then stratified by patient characteristics. All models were adjusted for age, diabetes, hypertension, and smoking.
To ensure that there was sufficient variability in ΔCCIS values, we conducted a case-controlled backward analysis. For this, the subjects were dichotomized into those who had a decrease or no change in CCIS over time (ΔCCIS ≤ 0); these were classified as “controls”; whereas those individuals in whom CCIS remained unchanged or increased (ΔCCIS > 0) were included in “cases”. Associations with continuous variables were determined by using unpaired t-testing and the results of both the forward (predictive) and backward (retrospective) analyses were then compared to identify common cell characteristics associated with improved outcomes.
All statistical analyses were conducted using the SAS 9.3 software (Cary, North Carolina). Because we prioritized knowledge discovery, no statistical techniques were employed to reduce the familywise error rate (e.g., Bonferroni correction). Thus, any results from these evaluations will need to be confirmed before they can be generalized to the larger subject population from which the sample was obtained.
Funding was provided by the National Heart, Lung, and Blood Institute (NHLBI) in conjunction with the CCTRN. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
In LateTIME, 87 patients were randomized to active treatment (58 subjects) or placebo (29 subjects). Three patients were excluded from the primary analysis in both the treatment (one did not receive cells, one was lost to follow-up, and one was without CMR data) and placebo (one death and two contraindications to CMR at follow-up) groups. The analyses reported below comprise all LateTIME subjects who had demographic and biorepository data; irrespective of whether they were treated with BMCs. The CCIS analysis is based on 73 patients who had both biorepository and LV function data.
Predictive Analysis
Cell and Patient Characteristics
To examine variability in the BM product at baseline, we assessed the association of patient characteristics with the abundance of specific cell populations in their BM. The association of patient characteristics with each of the 40 cell characteristics encompassing phenotypes and functional outgrowth assays was examined; however, Table 1 lists only cell populations exhibiting statistically significant associations with specific patient characteristics. As shown, older individuals were found to have higher levels and smokers were found to have lower levels of CD45+/CD3+ in their BM (Table 1). Smoking was associated with higher levels of CD34+/VEGFR2+/CD133+ (CD133+ includes CD45dimSSClowCD34+CD133+) cells, which are early, immature endothelial and hematopoietic progenitors. Subjects with high BMI had lower number of CD45dim/CD11bdim cells of hematopoietic origin. Older subjects and those with hypertension or elevated hs-CRP were found to have higher levels of CD45+/CD133+ cells. Subjects with low levels of hemoglobin also had lower levels of CD45+/CD34+ cells of hematopoietic origin in their BM.
Table 1.
Statistically significant patient characteristics above and below median cell phenotype values
| Patient Characteristics | Less than Median* | Greater than Median* | P-value |
|---|---|---|---|
| CD45+/CD3+ cells | |||
|
| |||
| Age Years (range) | 53.5 ± 11.9 [40] | 58.4 ± 9.7 [40] | 0.048 |
| Smoking | 75.0% [30] | 37.5% [15] | 0.001 |
|
| |||
| CD45+/CD133+ cells | |||
|
| |||
| Age Years (range) | 52.8 ± 10.9 [40] | 59.2 ± 10.5 [39] | 0.010 |
| Hypertension | 40.0% [16] | 64.1% [25] | 0.043 |
| HGB1† | 13.8 ± 1.4 [40] | 12.9 ± 1.8 [39] | 0.017 |
| hs-CRP‡ | 6.0 ± 7.6 [35] | 20.0 ± 34.8 [35] | 0.023 |
|
| |||
| CD45+/CD34+ cells | |||
|
| |||
| HGB1† | 13.8 ± 1.5 [40] | 12.9 ± 1.7 [39] | 0.009 |
|
| |||
| CD34+/VEGFR2+/CD133+ | |||
|
| |||
| Smoking | 40.5% [15] | 70.0% [28] | 0.012 |
|
| |||
| CD45dim/CD11bdim | |||
|
| |||
| Obesity (BMI§) | 29.6 ± 5.0 [39] | 26.5 ± 5.0 [39] | 0.008 |
Values represent mean ± SD. The number of individuals in each category is indicated in square parenthesis [n]. The gating strategy used for the identification of the indicated cell populations in the BM is shown in Supplemental Figure 1.
HGB1, Hemoglobin;
hs-CRP, High sensitivity C-reactive protein;
BMI, Body-mass index
To determine which patient characteristics are associated with functional properties of BMCs, we examined the association between patient characteristics with the colony-forming capacity of BMCs in CFU-Hill and ECFC-CFU assays. Among the patient characteristics examined, diabetes, hypertension, diuretic use, and hs-CRP levels were positively associated with the maximum number of MSC colonies present (Table 2). Smoking was negatively associated with MSC colony formation and diuretic use was significantly associated with lower number of maximal colonies formed in the CFU-EC assay. High hs-CRP levels were associated with higher number of total CD133 cells (%) in the product. These observations suggest that patient characteristics are related to not only the cell composition of the BM, but also its functional capacity in colony forming assays.
Table 2.
Statistically significant patient characteristics below or above the median outgrowth assays
| Functional Analysis | Below the Median* | Above the Median* | P-value |
|---|---|---|---|
| MSC† (maximum colony #) | |||
|
| |||
| Diabetes | 15.0% [6] | 36.6% [11] | 0.050 |
| Hypertension | 37.5% [15] | 70.0% [21] | 0.009 |
| Diuretic use | 17.5% [7] | 43.33 % [13] | 0.031 |
| hs-CRP‡ | 6.8 ± 7.6 [34] | 22.8 ± 38.5 [28] | 0.021 |
|
| |||
| MSC† colony | |||
|
| |||
| Smoking | 81.25% [13] | 37.5% [6] | 0.029 |
|
| |||
| CFU-EC§ colony max | |||
|
| |||
| Diuretic use | 41.3% [16] | 16.13% [5] | 0.035 |
|
| |||
| Total CD133 cell % | |||
|
| |||
| hs-CRP‡ | 6.3± 7.6 [39] | 22.2± 35.7 [37] | 0.008 |
Values represent mean ± SD or number (%). The number of individual in each category is indicated in square parenthesis [n].
MSC, Mesenchymal stromal cell;
hs-CRP, High sensitivity C-reactive protein;
CFU-EC, Colony-forming unit endothelial cell
Retrospective Analysis
Relationship between Cell Characteristics and Outcome
To determine whether BM phenotypes were associated with clinical outcomes, we examined the relationship between BMC populations and indices of cardiac function. Higher levels of CD45dim/CD11bdim cells were inversely related to LVEF and WMBZ, and positively associated with LVESVI (Table 3). BMC growth characteristics measured in outgrowth assays also were predictive of functional outcomes. Specifically, a higher ECFC colony forming capacity was associated with increased WMBZ and decreased LVESVI. Collectively, these observations suggest that characteristics of the BM, 2 to 3 weeks after STEMI, affect functional recovery of the heart. Specifically improvement may be compromised by BM comprised of higher levels of CD11bdim cells in the BM and reduced ECFC colony-forming capacity.
Table 3.
Change in left ventricular functional outcomes for unit difference in phenotype
| Variable* | Regression Coefficient ± SE | Confidence Interval for the Regression Coefficient | P-value | |
|---|---|---|---|---|
|
| ||||
| Lower boundary | Upper boundary | |||
| Change in ejection fraction | ||||
|
| ||||
| CD45dim/CD11bdim | −1.46 ± 0.56† | −2.55 | −0.36 | 0.012 |
| CD45dim/CD11bdim | −0.58 ± 0.21 | −1.0 | −0.16 | 0.010 |
|
| ||||
| Change in end systolic volume index | ||||
|
| ||||
| CD45dim/CD11bdim | 2.69 ± 0.92 | 0.89 | 4.5 | 0.005 |
| ECFC‡ colony | −11.36 ± 4.9 | −20.95 | −1.76 | 0.046 |
|
| ||||
| Change in border zone wall motion | ||||
|
| ||||
| CD45dim/CD11bdim | −0.43 ± 0.20 | −0.82 | −0.04 | 0.037 |
| ECFC‡ colony | 0.89 ± 0.33 | 0.24 | 1.53 | 0.022 |
Variable is either cell phenotype or cell functional analysis as indicated. The gating strategy used for the identification of the indicated cell populations in the BM is provided in Supplemental Figure 2.
Patients whose CD45+/CD11b+ is one unit greater than others will have on average a 1.46 decrease in ejection fraction;
ECFC, Endothelial colony-forming cell
Association of LV function parameters with cell characteristics
We examined whether improvements in measures of cardiac function were associated with cell characteristics. We found that after adjusting for age, hypertension, diabetes, smoking, creatine kinase-MB (CKMB), and angiotensin-converting enzyme (ACE) inhibitor use and baseline LVEF, CD45dim/CD11bdim cells were negatively associated with global LVEF (P <0.02), as well as both WMIZ (P <0.001), and WMBZ (P <0.01), 6 months after infusion. In addition, global LVEF was positively associated with MSC colony formation (P <0.02) and WMBZ with percent cell viability (P <0.03) (Table 3). When only the patients receiving cell therapy were examined, we found negative associations between CD45dim/CD11bdim cells and LVEF (P<0.02), LVESV (P<0.002) and WMBZ (P<0.03) after adjusting for age, diabetes, hypertension and smoking. Negative associations were also observed between CD45dim/CD11bright cells and LVEF (P<0.002), WMIZ (P<0.007) and WMBZ (P< 0.02), indicating that specific CD11b+ cell populations are associated with poor outcomes in patient irrespective of cell therapy.
Association of CCIS with Patient Characteristics and Comorbidity
To assess the relationship of CCIS to cardiac performance, baseline characteristics of patients stratified by CCIS are presented in Table 4. Of 73 individuals, 37 had CCIS values below the median and 36 had CCIS values above the median. Patients with CCIS values above the median tended to have on lower screening LVEF by echocardiography in comparison with those with values below the median (33.5 ± 6.3, vs. 37.8 ± 6.4, P = 0.005), higher peak CKMB (363.2 ± 265.4 vs. 189.5 ± 156.1, p = 0.005), and higher troponin levels (for troponin T, 11.4 ± 6.5 vs. 5.3 ± 4.8, P = 0.011). They were also more likely to be on diuretics at baseline (33.3% vs 3%, P = 0.01).
Table 4.
Baseline Characteristics of Median Baseline Cardiac Composite Impairment Score (CCIS)
| Low CCIS* (n=37) | High CCIS* (n=36) | P-value | |
|---|---|---|---|
| Age | 58.4 ±11.4 | 53.7 ±11.7 | 0.080 |
| Male | 31 (83.78%) | 31 (86.11%) | 1.000 |
| Race (white) | 32(86.49%) | 32(88.89%) | 1.000 |
| Diabetes | 5(13.51%) | 8(22.22%) | 0.374 |
| Hypertension | 17(45.95%) | 21(58.33%) | 0.352 |
| Hyperlipidemia | 26(70.27%) | 25(69.44%) | 1.000 |
| Angina | 10(27.03%) | 5(13.89%) | 0.247 |
| Smoking | 19(51.35%) | 23(63.89%) | 0.346 |
| Pre-infarction Angina | 11(30.56%) | 9(25.00%) | 0.793 |
| BMI† | 27.8 ± 4.6 | 27.0 ±4.4 | 0.467 |
|
| |||
| Peak CKMB‡ | 189.5 ±156.1 | 363.2 ±265.4 | 0.005 |
| Peak Troponin T1 | 5.3 ± 4.8 | 11.4 ±6.5 | 0.011 |
| Peak Troponin I1 | 71.4 ± 58.6 | 233.4 ± 215.6 | 0.025 |
|
| |||
| Door to Balloon Time | 4.0 ± 6.8) | 9.4 ± 26.9 | 0.245 |
| ACE§ inhibition | 28(75.68%) | 26(72.22%) | 0.794 |
| Anti-angina therapy | 5(13.51%) | 3(8.33%) | 0.711 |
| Anticoagulants | 8(21.62%) | 12(33.33%) | 0.302 |
| Non Aspirin Antiplatelet | 29(78.38%) | 34(94.44%) | 0.085 |
| Aspirin | 36(97.30%) | 34(94.44%) | 0.615 |
| Beta blockers | 30(81.08%) | 34(94.44%) | 0.152 |
| Calcium Channel Blockers | 0(0.0%) | 1(2.78%) | 0.493 |
| Cholesterol Lowering | 35(94.59%) | 35(97.22%) | 1.000 |
|
| |||
| Diuretic use | 3(8.11%) | 12(33.33%) | 0.010 |
|
| |||
| Insulin | 2(5.41%) | 2(5.56%) | 1.000 |
| Nitrates | 12(32.43%) | 14(38.89%) | 0.630 |
| Oral hypoglycemics | 3(8.11%) | 3(8.33%) | 1.000 |
CCIS, Composite Cardiac Impairment Score; A low CCIS corresponds to less LV dysfunction at baseline (i.e., better LV function), while the converse is true for a high CCIS.
BMI, Body-mass index;
CKMB, creatine kinase-MB;
ACE, Angiotensin-converting enzyme
Association of CCIS with Cell Characteristics
Of the 73 subjects, 40 improved their CCIS score over 6 months and 33 experienced a worsening of the score. To examine which cell populations were associated with CCIS, adjusted multivariate analyses were performed to assess the relationship between the cell phenotype or the functional assay and changes over 6 months both the traditional outcome variables and the CCIS.
Association of CCIS with Clinical Outcomes
Multivariable analysis of those with high CCIS (cases) compared with those with low CCIS (controls) revealed a significant association of several cell phenotypes with a change in CCIS (Table 5). Specifically, BM levels of CD133+, CD31+/CD34+, CD45dim/CD34+ and CD45+/CXCR4dim cells were higher in individuals who showed an improvement in the CCIS score. The levels of CD45dim/CD11bdim cells were higher in the BM of individuals that showed worsened CCIS 6 months after study product delivery. Individuals showing an improvement in their CCIS also had higher levels of ECFC colonies in the outgrowth assay. Collectively, these data suggest that individuals with higher levels of CD34+ and CD133+ cells in their BM are likely to show more LV functional improvement after STEMI, whereas those with higher levels of CD11bdim cells show poorer outcomes.
Table 5.
Association of Phenotypes and Functional Outcomes with Cardiac Composite Impairment Score (CCIS)
| Variable* | Improved (lower) CCIS† (n=40) | Worsened (higher) CCIS (n=33) | P-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean ± SD [n] | MIR‡ | Mean ± SD [n] | MIR | Unadj | Adj* | |
| CD133+ | 1.3 ± 0.5 [37] | 1.2 (0.9–1.6) | 1.0 ± 0.4 [30] | 1.1 (0.6–1.3) | 0.027 | 0.012 |
| CD31+/CD34+ | 3.0 ±1.6 [37] | 2.5 (1.9–3.8) | 2.1 ±1.0 [29] | 1.9 (1.5–2.9) | 0.013 | 0.012 |
| CD45dim/CD34+ | 2.8 ±1.1 [37] | 2.5 (2.1–3.6) | 2.2 ±1.0 [30] | 2.0 (1.6–2.7) | 0.021 | 0.013 |
| CD45dim/CD11bdim | 2.0 ±1.2 [36] | 1.8 (0.9–2.7) | 3.1 ±2.0 [29] | 2.5 (1.8–4.4) | 0.006 | 0.004 |
| CD45+/CXCR4dim | 3.1 ±1.4 [36] | 2.8 (2.0–3.9) | 2.1 ±1.5 [29] | 1.5 (1.2–2.5) | 0.012 | 0.009 |
| CFU-EC§/Dose | 255 ± 337 [32] | 135 (0.0–345) | 109 ±142 [26] | 60 (0.0–90.0) | 0.045 | 0.068 |
The gating strategy used for the identification of the indicated cell populations in the BM is shown in Supplemental Figure 3.
CCIS, Composite Cardiac Impairment Score;
MIR, Median Interquartile Range, adjusted; for diabetes, hypertension, and smoking and treatment assignment;
CFU-EC, Colony-forming unit endothelial cell
Discussion
The major findings of this study are that in the LateTIME trial, the composition of the BM varied with the characteristics of STEMI patients and that the improvements in cardiac performance after BM infusion were associated with the frequency of CD133+ and CD34+ cell populations within the BM at baseline, independent of cell therapy. In addition, both predictive and dichotomous analyses indicate that high levels of CD11bdim cells in the BM were associated with an inferior clinical outcome and the levels of these cells were negatively associated with conventional indices of cardiac function (LVEF, WMIZ, and WMBZ). These findings from the LateTIME trial support the novel concept that the efficacy of stem cell therapy is significantly modified by both stem cells and non-stem cells present in the BM. Further elucidation of the role individual BM cell populations in cell therapy in other trials could significantly advance our understanding of the basic biology of this therapeutic intervention and provide new approaches for improving its efficacy.
Previous work has shown that the levels of circulating stem/progenitor cells are associated with CVD risk factors 13, and predict the occurrence of cardiovascular events and death from cardiovascular causes 14, however, the association between cardiovascular disease risk and BM characteristics has been seldom studied. In most cell therapy studies BM is aspirated after MI, which acutely increases the levels of specific stem/progenitor cells in the peripheral blood 15–17. Because these cells arise from the BM, injury-dependent recruitment could significantly alter BMC as well as their functional characteristics. Indeed our recent analysis shows that STEMI is associated with a decrease in the endothelial colony-forming capacity of BMCs and the levels 18 of CD34+ cells (including CD45dimSSClow, CD34+ cells) reach a nadir in the BM 7 days after STEMI 18. In the LateTIME trial, in which BM was aspirated 2–3 weeks after STEMI, BM characteristics could be evaluated, without confounding by acute inflammatory events post-MI. Hence, the association between BMC constituents and patient demographic features such as age, hypertension, obesity, and smoking observed in our study reflects the effects of cardiovascular disease risk factors on BMC constituents independent of acute changes post-MI. Moreover, by identifying an association between positive functional outcomes and the BM abundance of CD133+, CD34+, and negative outcomes with high levels of CD11bdim cells, these results extend our previously reported observation that BMC characteristics at baseline are associated with better clinical outcomes of BMC therapy 9.
The CD11b+ cells in the BM represent a collection of inflammatory cells. CD11b is the α-subunit of the CD11b/CD18 heterodimer involved in the adhesion of monocytes, granulocytes, and natural killer cells 19. In animal studies, acute myocardial injury and ischemia recruit BM-derived CD11b+ cells through a VEGF/SDF-1 signaling, a mechanism important for tissue angiogenesis and increased perfusion in response to injury 20. In chronic heart failure, inhibition of the recruitment of CD11b+ cells from the spleen to the heart prevents pathological remodeling and inflammation 21, furthermore, sustained infiltration of CD11b+ in the heart is associated with chronic inflammation, fibrosis, and myocyte atrophy 22. In our analysis, CD11bdim cells were associated with less favorable outcomes. These results are in accordance with the animal data and suggest that BM CD11b+ cells may be important in supporting tissue repair and play diverse roles in the injury response, depending upon the severity of the injury. However, additional studies will be required to fully understand the role of BM-derived CD11b+ cells and to delineate their contribution to myocardial recovery and stem cell therapy.
We found a positive association between the levels of CD133+ and CD34+/CD31+ cell populations and improved clinical outcomes. CD133+ is a well-conserved glycoprotein antigen that has been used to identify early immature endothelial and hematopoietic progenitors 23,24. In vitro, CD133+ cells differentiate into mature endothelial cells 23,24, and they secrete a variety of trophic growth factors (FGF, VEGF, IL-10, IGF) and matrix-remodeling factors, such as metalloproteinases and their inhibitors that can reduce tissue damage and promote tissue healing 25,26. In animal models of AMI, transplantation with CD133+ cells has been found to reduce fibrosis and myocyte apoptosis and to improve cardiac function 23,27. Multiple clinical trials testing the efficacy of CD133+ cells in restoring cardiac function after STEMI 28 or in patients with refractory angina 29 and ischemic cardiomyopathy 30 have been reported. These trials, most of which are small, have shown variable results, with some 28,29 showing significant and sustained improvement in cardiac function, while others 31 report marginal or no improvement. Our observation that the frequency of CD133+ cells in the BM is associated with improved CCIS supports the view that these cells are important constituents of the BM that could favorably affect myocardial recovery after STEMI. However, we also observed that the levels of CD133+ varied in accordance with age, hypertension, smoking, screening LVEF, hemoglobin, and hs-CRP levels (Table 1). Hence, the benefits cell therapy may be confounded by the variable levels of CD133+ cells in the patient BM product. Careful patient selection may be required to identify those with high levels of CD133+ in the BM.
In previous studies, decreased circulating levels of CD133+ have been associated with several CVD risk factors. In particular, it has been shown that individuals with high values of composite Framingham risk score 13, or CVD risk factors, such as obesity 32, diabetes 33, pulmonary hypertension 34, and endothelial dysfunction 35, have lower levels of circulating CD133+ cells. In contrast, we found that in the BM, age, hypertension, smoking, and hs-CRP levels were all positively associated with CD133+ cells. These observations suggest that while CVD risk factors are associated with a decrease in the levels of these cells in the blood, there is an increase in the levels of these cells in the BM. This differential effect may be due to a defect in the mobilization of these cells from the BM to the peripheral blood due to age- or disease-dependent resistance to mobilization signaling triggered by VEGF or SDF-1 or may reflect an increased production commensurate with increased trafficking of the cells. In future studies it would be of interest to determine which specific recruitment signals are attenuated and whether the mobilization and recruitment of these cells improve myocardial recovery after injury, independent of exogenous cell therapy.
Most clinical trials to date have used either unfractionated BM or BM preparations positively selected for CD34+ or CD133+ cells; but negative selection of the BM to deplete specific cell populations may provide a new strategy for improving potential outcomes. Preclinical studies in mice have shown that injection of CD34+ cells alone after MI results in better functional recovery and decreased fibrosis than unfractionated mononuclear cells containing similar number of CD34+ cells 36, suggesting that the unfractionated mononuclear cell population may contain a “negative effector” cell population that attenuates the beneficial effects of CD34+ cells. Our studies suggest that the CD11bdim cells may be one potential candidate for a “negative effector cell” that could be selectively removed from the cell product before therapy to enhance functional outcomes.
The present results also provide additional criteria for post-manufacturing validation of cell potency data. As indicated by our analysis of CCIS, higher levels of ECFC colonies in the outgrowth assays were associated with better clinical outcomes in patients after STEMI; therefore, selection of patients with higher ECFC colony yields may be useful in optimizing BM therapy and targeting recruitment of specific patient populations. Similarly, additional patient selection criteria could be developed for selection or exclusion of patients with comorbidities such as diabetes, smoking, or diuretic use in order to fully redeem the promise of cell therapy, albeit in a more restricted patient population.
Limitations of the analysis of the associations between BM product characteristics and functional outcomes have been noted previously for the TIME-CCTRN trial 9 and are applicable here. In addition, while we prospectively stated that an evaluation of the relationship between BM phenotypes and LV function would be carried out, we had considerable freedom in determining the analysis strategy. Therefore, two assessments were made. The first was a predictive analysis that looked forward from baseline phenotypes to changes in CCIS. The advantage of this analysis is that it addresses the most clinically relevant question of whether baseline phenotypes can predict improvement in CCIS. The disadvantage of this approach is that in a trial that did not meet its primary objective, there may not be enough variability in CCIS to uncover a relationship between baseline phenotypes and outcomes. For this reason, we augmented our assessments with a backward analysis, which enabled us to segregate patients by the extent of change of CCIS. When we compared the baseline phenotype characteristics of the two cohorts, we identified several phenotypes whose values were different between these two groups. Finally, the large number of P values with no attendant correction for multiplicity admits a high probability of at least one relationship being attributable to sample error. While we cannot completely rule out this possibility, the concordance between our retrospective and prospective analyses, similarity of results in patient who did or did not receive cell therapy, and the agreement of our findings with previous reports, particularly in regard to the role of CD34+ and CD133+ cells suggests that these and other findings of the study cannot be regarded as being solely due to multiple testing. Nevertheless, these results must be confirmed by similar analyses of other datasets before they can be generalized
Conclusions
Our analysis of subjects from the LateTIME-CCTRN cohort has disclosed new associations between BMC characteristics and patient characteristics and between CD11bdim cells, while confirming established relationships involving CD133+ and CD34+ phenotypes. If confirmed in other studies, these results would provide a potential rationale for modifying the composition of BM products to maximize its therapeutic efficacy. Results indicating that the composition of the BM varied with CVD risk factors and suggest that mobilization of stem/progenitor cell population may be impaired in high-risk patients. These results are likely to inform the design of future clinical trials to optimize cell therapy and target cell therapy to specific patient populations.
Supplementary Material
Acknowledgments
The opinions expressed by one of the authors (RFE) do not reflect those of the National Institutes of Health, the US Dept. of Health and Human Services, or the US Government.
Funding Source: This work was supported by the National Heart, Lung, and Blood Institute under cooperative agreement 5 UM1 HL087318.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duran JM, Makarewich CA, Sharp TE, et al. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113(5):539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burchfield JS, Iwasaki M, Koyanagi M, et al. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. 2008;103(2):203–211. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 3.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delewi R, Hirsch A, Tijssen JG, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: A collaborative meta-analysis. Eur Heart J. 2014;35(15):989–998. doi: 10.1093/eurheartj/eht372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surder D, Manka R, Lo Cicero V, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: Effects on global left ventricular function. Circulation. 2013;127(19):1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 7.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cogle CR, Wise E, Meacham AM, et al. Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115(10):867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutt RC, Trachtenberg BH, Cooke JP, et al. Bone marrow characteristics associated with changes in infarct size after STEMI: A biorepository evaluation from the CCTRN TIME trial. Circ Res. 2015;116(1):99–107. doi: 10.1161/CIRCRESAHA.116.304710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zierold C, Carlson MA, Obodo UC, et al. Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular cell therapy research network biorepository core laboratory rationale. Am Heart J. 2011;162(6):973–980. doi: 10.1016/j.ahj.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolliffe I. Principal component analysis. 2. Aberdeen: Springer; 2002. [Google Scholar]
- 12.Ringner M. What is principal component analysis? Nat Biotechnol. 2008;26(3):303–304. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110(4):624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 15.Leone AM, Rutella S, Bonanno G, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26(12):1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 16.Massa M, Rosti V, Ferrario M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 17.Wojakowski W, Tendera M, Michalowska A, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110(20):3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 18.Cogle CR, Wise E, Meacham AM, et al. A detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.305422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahl HL, Rosmarin AG, Tenen DG. Characterization of the myeloid-specific CD11b promoter. Blood. 1992;79(4):865–870. [PubMed] [Google Scholar]
- 20.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res. 2014;114(2):266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier HJ, Schips TG, Wietelmann A, et al. Cardiomyocyte-specific IkappaB kinase (IKK)/NF-kappaB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2012;109(29):11794–11799. doi: 10.1073/pnas.1116584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma N, Ladilov Y, Moebius JM, et al. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: Bone marrow vs. cord blood-derived cells. Cardiovasc Res. 2006;71(1):158–169. doi: 10.1016/j.cardiores.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Suuronen EJ, Veinot JP, Wong S, et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(1 Suppl):I138–44. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 25.Barcelos LS, Duplaa C, Krankel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of wnt signaling. Circ Res. 2009;104(9):1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratajczak J, Kucia M, Mierzejewska K, et al. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells--implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22(3):422–430. doi: 10.1089/scd.2012.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 28.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation. 2005;112(9 Suppl):I178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 29.Adler DS, Lazarus H, Nair R, et al. Safety and efficacy of bone marrow-derived autologous CD133+ stem cell therapy. Front Biosci (Elite Ed) 2011;3:506–514. doi: 10.2741/e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361(9351):45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 31.Nasseri BA, Ebell W, Dandel M, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: The Cardio133 trial. Eur Heart J. 2014;35(19):1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 32.Muller-Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction. Eur Heart J. 2008;29(12):1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 33.Fadini GP, de Kreutzenberg SV, Coracina A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27(18):2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 34.Smadja DM, Gaussem P, Mauge L, et al. Circulating endothelial cells: A new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation. 2009;119(3):374–381. doi: 10.1161/CIRCULATIONAHA.108.808246. [DOI] [PubMed] [Google Scholar]
- 35.Werner N, Wassmann S, Ahlers P, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102(6):565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 36.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114(20):2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


