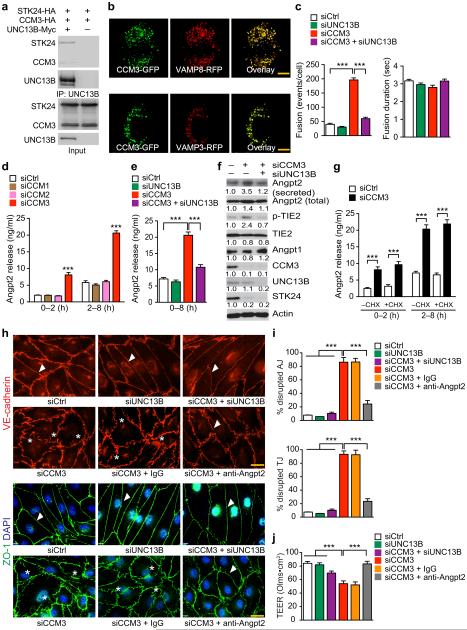
Fig.3. CCM3 restrains ANGPT2 release from ECs and maintains EC junctions.
a. UCN13B forms a complex with CCM3 and STK24. Association of UNC13B with CCM3-STK24 was determined by co-immunoprecipitation with anti-Myc (UNC13B) followed by Western blotting with anti-HA for CCM3 and STK24. b. Co-localization of CCM3-GFP and VAMP8-RFP or VAMP3-RFP expressed in HBMVECs after transient transfection. Representative images were shown for 1 of 10 cells examined. c. CCM3-deficiency increases basal exocytic fusion events (but not fusion duration) which were rescued by co-silencing UNC13B. n=10, **P<0.01 (one-way ANOVA). d–g. CCM3 regulates ANGPT2 secretion in ECs. HBMVECs were transfected with siRNAs for 72 hours followed by treatment with CHX (in panel g) for 2–8 h. ANGPT2 levels in media were determined by ELISA. Secreted ANGPT2 and intracellular proteins were detected by Western blotting. Data in panel f represent fold changes with control siRNA normalized to 1.0. n=6, **P <0.01 (two-way ANOVA in panels d and g; one-way ANOVA in panel e). h–j. CCM3-ANGPT2 axis regulates EC junctions and permeability. A control IgG or ANGPT2 neutralizing antibody (10 μg/ml) was added to HBMVECs at 16 h post-transfection with siRNAs. EC adherens junctions (AJ) and tight junctions (TJ) (h) were visualized by immunostaining, and percentages of disrupted junctions are quantified in panel i (counting 100 microscope fields in each group). j. Barrier function of ECs cultured on fibronectin-coated ECIS cultureware was assessed for transendothelial electric resistance (TEER; expressed as OLMS multipled by cm2) by electrical cell-substrate impedance sensing. All experiments were repeated twice. Error bars indicate s.e.m., n=12. **P < 0.01 (one-way ANOVA). Scale bar: b and h: 20 μm.