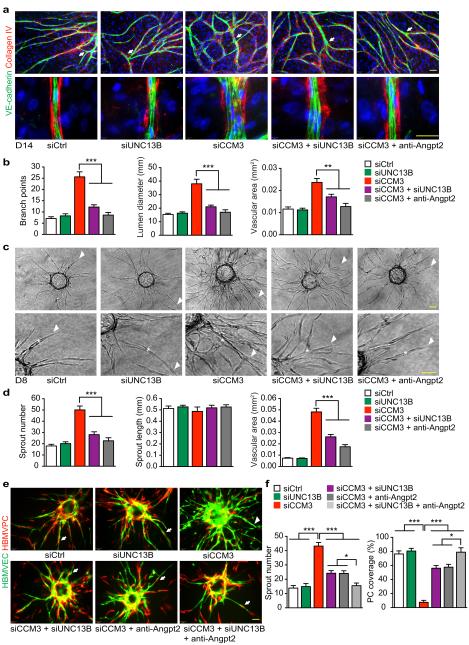
Fig.4. CCM3 maintains normal EC lumen formation and EC-PC associations.
a–b. Organotypic angiogenesis assay. HBMVECs were transfected with siRNAs. 24h after transfection, cells were seeded onto a confluent layer of fibroblasts and were co-cultured for 14 days in the absence or presence of anti-ANGPT2 (10 μg/ml). EC sprouts and lumens were visualized by VE-cadherin and collagen IV staining. Branches are indicated by arrows. A representative high power confocal image for tubule lumen from each group is shown (a). Number of branch point, mean lumen diameter and lumen areas are quantified (b). c–d. 3D spheroid sprouting assay. siRNA-transfected HBMVECs were coated with microbeads, embedded in fibrin gels and grown in EGM2 medium for 8 days. A representative image of 10 beads for each sample is shown with sprouts and lumens indicated by arrowhead and asterisk, respectively. Quantifications of sprout number, sprout length and lumen areas are shown in panel d. e–f. EC-PC interactions in 3D spheroid sprouting assay. HBMVECs were infected with EGFP-expressing retroviruses and HBMVPCs were infected with mCherry-expressing lentiviruses. ECs were further transfected with siRNAs. ECs and PCs (2:1 ratio) were seeded to beads and treated as described in panels c–d. EC sprouts and PC coverage were visualized. A representative image of 10 beads for each sample is shown in panel e and % PC coverage of sprouts are quantified in panel f. n=10, *P <0.05; **P <0.01 (one-way ANOVA). Additional two independent experiments were performed. Error bars indicate s.e.m. Scale bar: 100 μm.