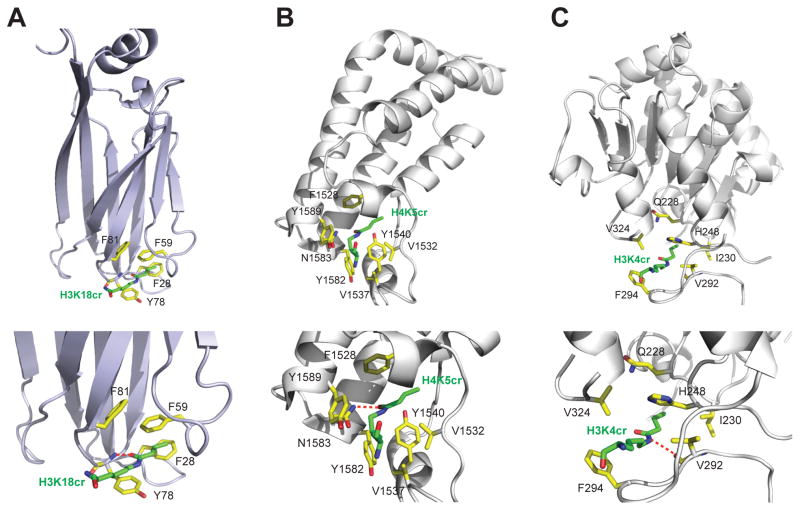
Figure 3. Crotonyl-lysine recognition by distinct histone binding modules.
(A) H3K18cr recognition by the AF9 YEATS domain, as depicted in the overall protein structure (upper) and the detailed interactions at the crotonyl-lysine binding site (lower).
(B) H4K5cr recognition by the second bromodomain of TAF1 (TAF1-BD2), as shown in the crystal structure of the TAF1_BD2/H4K5cr complex (PDB: 4YYN). The side-chain carbonyl of the crotonyl-lysine H4K5cr is hydrogen-bonded to the conserved Asn1583, similar to what’s seen for acetyl-lysine recognition by the bromodomain.
(C) H3K4cr recognition by Sirt3, as shown in the crystal structure of human Sirt3/H3K4cr complex (PDB: 4V1C). The side-chain amide of crotonyl-lysine is hydrogen-bonded to the backbone carbonyl of Val292 of the protein.