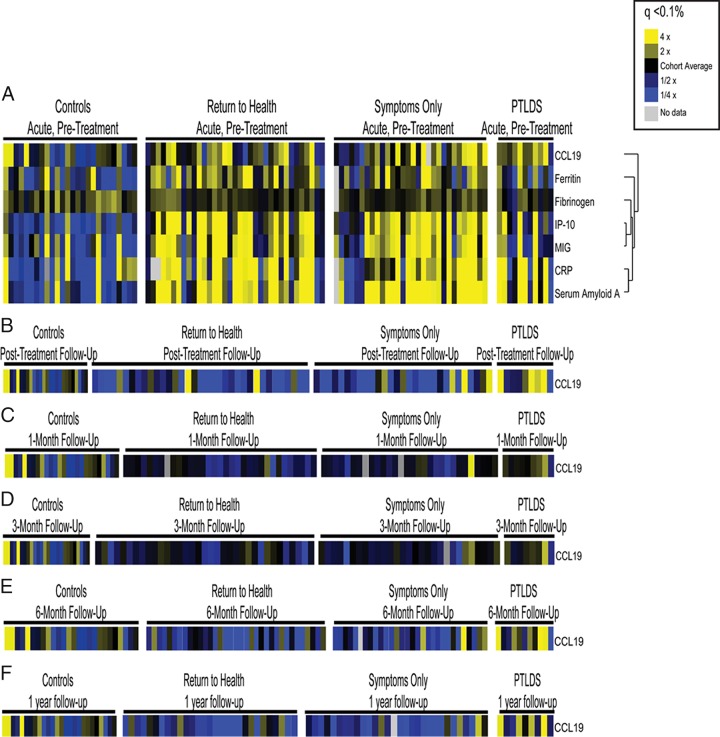
FIG 2.
Identification of relevant immune mediators. Serum samples from participants with early Lyme disease and healthy controls were assayed for the presence of soluble mediators using an optimized multiplex-based assay system. Mediators with significant changes (q < 0.1%) are displayed as heat maps to visualize differences. All study time points are represented in panels A to F for healthy controls, those that returned to health, those that reported symptoms without functional impact, and those that met the criteria for PTLDS (25). (A) Mediators with significant changes (q < 0.1%) at the acute-phase, pretreatment visit; (B) mediators with significant changes (q < 0.1%) at the posttreatment follow-up visit 3 weeks later; (C) mediators with significant changes (q < 0.1%) at 1 month following treatment completion; (D) mediators with significant changes (q < 0.1%) at 3 months following treatment completion; (E) mediators with significant changes (q < 0.1%) at 6 months following treatment completion; (F) mediators with significant changes (q < 0.1%) at 1 year following treatment completion. CRP, C-reactive protein.