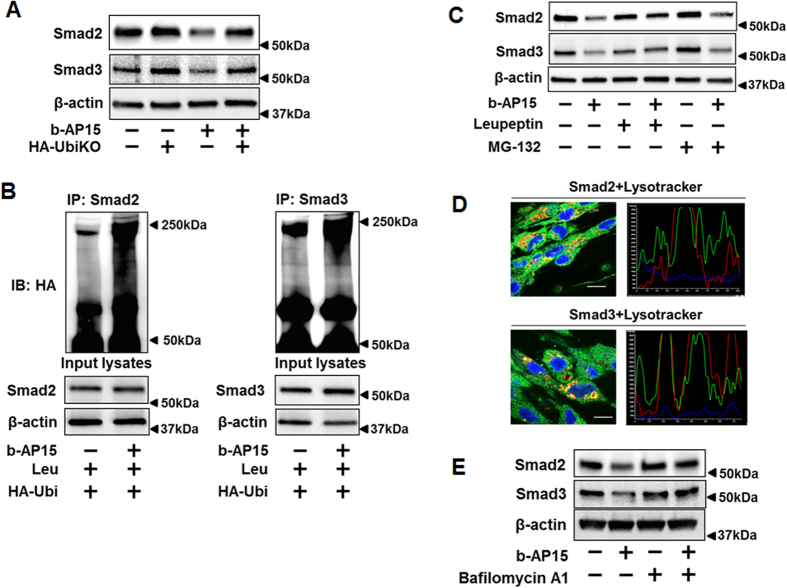
Figure 3. b-AP15 increases poly-ubiquitination of Smad2/Smad3 and degradation in lysosome.
(A) HLF cells were transfected with empty vector or HA-UbiKO plasmid, and then cells were treated with b-AP15 (10 μM) for 1 h. Cell lysates were analyzed by immunoblotting with antibodies to Smad2, Smad3, and β-actin. (B) HLF cells were transfected with HA-tagged ubiquitin (HA-Ubi) for 48 h, and then treated with leupeptin (100 μM) for 2 h, followed DMSO and b-AP15 (10 μM) treatment for additional 1 h. Cell lysates were subjected to immunoprecipitation with Smad2 or Smad3 antibody, followed by immunoblotting with HA tag antibodies. Input cell lysates were analyzed by immunoblotting with antibodies to Smad2, Smad3, and β-actin. (C) HLF cells were pretreated with leupeptin (100 μM) or MG-132 (20 μM) for 2 h, then treated with b-AP15 (10 μM) for 1 h. Cell lysates were analyzed by immunoblotting with antibodies to Smad2, Smad3, and β-actin. (D) HLF cells were pretreated with bafilomycin A1 (10 μM) for 1 h, then cells were treated with b-AP15 (10 μM) for 1 h. Cells were fixed with 3.7% formaldehyde. Cellular location of Smad2, Smad3, and lysosome were detected by immunostaining with antibodies to Smad2 and Smad3, and lysotracker. DAPI was used for nuclei staining (blue). Scale bar, 10 μm. 94.5% of the cells with double staining show positive co-localization. (E) HLF cells were pretreated with bafilomycin A1 (10 μM) for 1 h, then cells were treated with b-AP15 (10 μM) for 1 h. Cell lysates were analyzed by immunoblotting with antibodies to Smad2, Smad3, and β-actin. Western blot images were cropped to improve the conciseness of the data; samples derived from the same experiment and the blots were processed in parallel. Representative of experiments performed at least 3 independent times.