Abstract
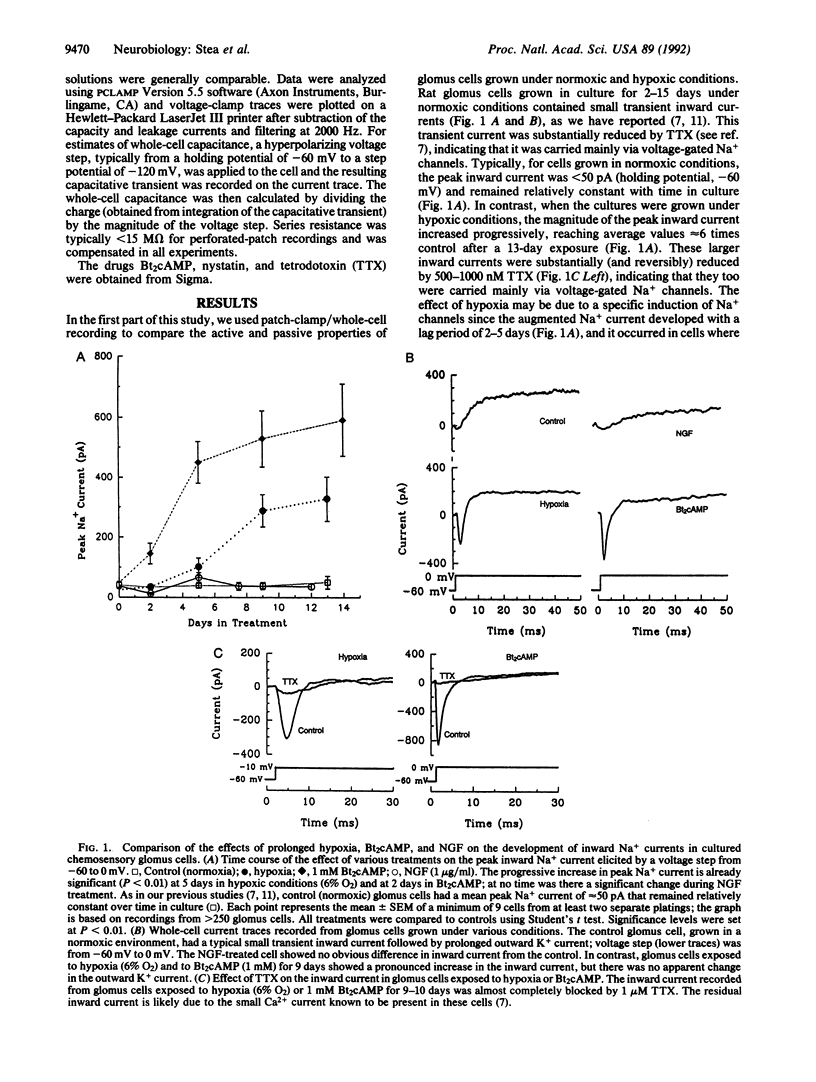
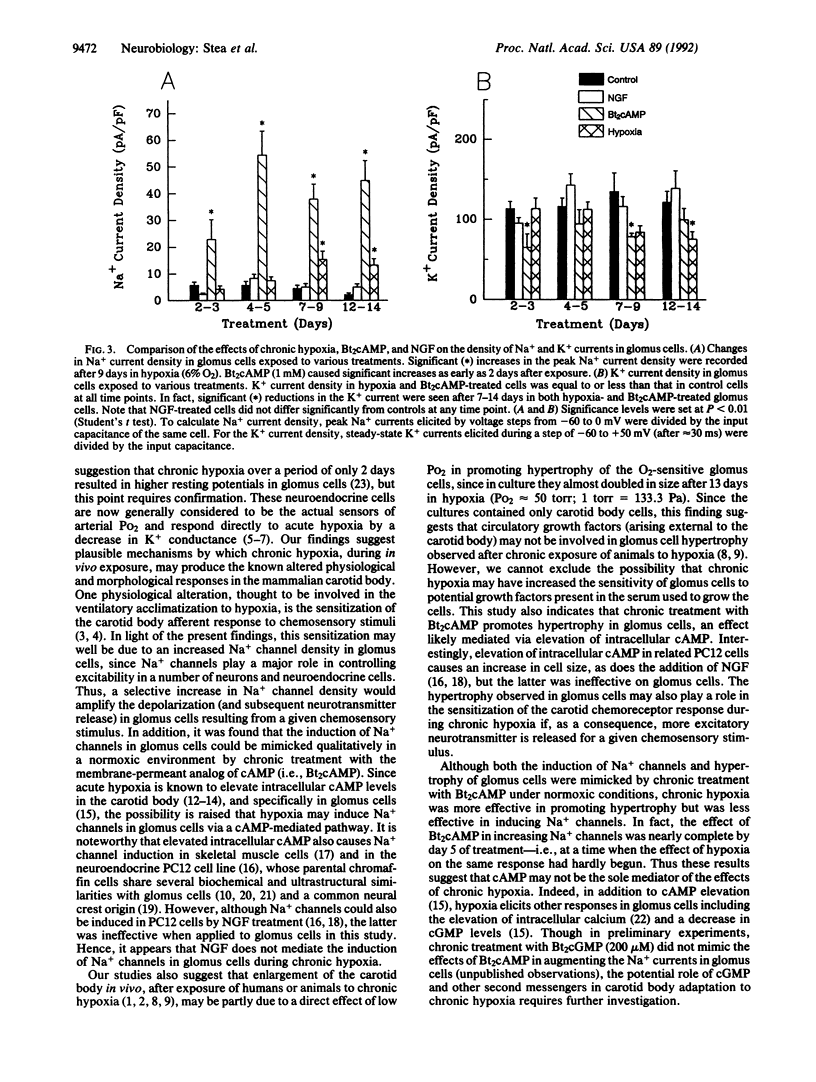
Chronic hypoxia sensitizes the ventilatory reflex in mammals and causes enlargement of the carotid body, a peripheral arterial chemosensory organ. To investigate possible underlying mechanisms, in the absence of circulatory changes, we exposed cultures of dissociated rat carotid body containing the oxygen sensors (i.e., chromaffin-like glomus cells) to chronic hypoxia (6% O2) over a period of 2 weeks. After a delay of a few days, the Na+ current density in hypoxia-treated glomus cells increased significantly, reaching values up to 6 times that seen in normoxic (20% O2) controls. In addition the whole-cell capacitance, an indicator of cell size, was also significantly larger (3-4 times control) in glomus cells exposed to chronic hypoxia. Both effects were mimicked qualitatively by chronic treatment of normoxic cultures with N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate, but not nerve growth factor, which is known to induce similar changes in the chromaffin cell line PC12. Thus, the physiological and morphological effects of chronic hypoxia on the carotid body in vivo may be due in part to a cAMP-mediated stimulation of Na+ channel expression and hypertrophy in the chemosensory glomus cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Pietruschka F. Membrane potential of cultured carotid body glomus cells under normoxia and hypoxia. Brain Res. 1984 Oct 8;311(1):148–151. doi: 10.1016/0006-8993(84)91408-2. [DOI] [PubMed] [Google Scholar]
- Aloe L., Levi-Montalcini R. Comparative studies on the effects elicited by pre- and postnatal injections of anti-NGF, guanethidine, and 6-hydroxydopamine in chromaffin and ganglion cells of the adrenal medulla and carotid body in infant rats. Adv Biochem Psychopharmacol. 1980;25:221–226. [PubMed] [Google Scholar]
- Barnard P., Andronikou S., Pokorski M., Smatresk N., Mokashi A., Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol (1985) 1987 Aug;63(2):685–691. doi: 10.1152/jappl.1987.63.2.685. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Acker H. Hypoxia increases the cyclic AMP content of the cat carotid body in vitro. J Neurochem. 1991 Jul;57(1):291–297. doi: 10.1111/j.1471-4159.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Dhillon D. P., Barer G. R., Walsh M. The enlarged carotid body of the chronically hypoxic and chronically hypoxic and hypercapnic rat: a morphometric analysis. Q J Exp Physiol. 1984 Apr;69(2):301–317. doi: 10.1113/expphysiol.1984.sp002807. [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Landis S. C., Patterson P. H. Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985 Aug;5(8):2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Heath D., Harris P., Castillo Y., Krüger H., Arias-Stella J. The carotid body in animals at high altitude. J Pathol. 1971 Aug;104(4):231–238. doi: 10.1002/path.1711040404. [DOI] [PubMed] [Google Scholar]
- Edwards C., Heath D., Harris P. The carotid body in emphysema and left ventricular hypertrophy. J Pathol. 1971 May;104(1):1–13. doi: 10.1002/path.1711040102. [DOI] [PubMed] [Google Scholar]
- Kalman D., Wong B., Horvai A. E., Cline M. J., O'Lague P. H. Nerve growth factor acts through cAMP-dependent protein kinase to increase the number of sodium channels in PC12 cells. Neuron. 1990 Mar;4(3):355–366. doi: 10.1016/0896-6273(90)90048-k. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- McGregor K. H., Gil J., Lahiri S. A morphometric study of the carotid body in chronically hypoxic rats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Nov;57(5):1430–1438. doi: 10.1152/jappl.1984.57.5.1430. [DOI] [PubMed] [Google Scholar]
- Nielsen A. M., Bisgard G. E., Vidruk E. H. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol (1985) 1988 Oct;65(4):1796–1802. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- Nurse C. A. Carbonic anhydrase and neuronal enzymes in cultured glomus cells of the carotid body of the rat. Cell Tissue Res. 1990 Jul;261(1):65–71. doi: 10.1007/BF00329439. [DOI] [PubMed] [Google Scholar]
- Offord J., Catterall W. A. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989 May;2(5):1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Rost F. W., Fontaine J., Le Lièvre C., Le Douarin N. Demonstration of the neural crest origin of type I (APUD) cells in the avian carotid body, using a cytochemical marker system. Histochemie. 1973;34(3):191–203. doi: 10.1007/BF00303435. [DOI] [PubMed] [Google Scholar]
- Pollock J. D., Krempin M., Rudy B. Differential effects of NGF, FGF, EGF, cAMP, and dexamethasone on neurite outgrowth and sodium channel expression in PC12 cells. J Neurosci. 1990 Aug;10(8):2626–2637. doi: 10.1523/JNEUROSCI.10-08-02626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Effects of different types of stimulation on cyclic AMP content in the rabbit carotid body: functional significance. J Neurochem. 1990 Oct;55(4):1287–1293. doi: 10.1111/j.1471-4159.1990.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Stea A., Alexander S. A., Nurse C. A. Effects of pHi and pHe on membrane currents recorded with the perforated-patch method from cultured chemoreceptors of the rat carotid body. Brain Res. 1991 Dec 13;567(1):83–90. doi: 10.1016/0006-8993(91)91439-8. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Wang W. J., Cheng G. F., Yoshizaki K., Dinger B., Fidone S. The role of cyclic AMP in chemoreception in the rabbit carotid body. Brain Res. 1991 Feb 1;540(1-2):96–104. doi: 10.1016/0006-8993(91)90495-h. [DOI] [PubMed] [Google Scholar]
- Wang Z. Z., Stensaas L. J., de Vente J., Dinger B., Fidone S. J. Immunocytochemical localization of cAMP and cGMP in cells of the rat carotid body following natural and pharmacological stimulation. Histochemistry. 1991;96(6):523–530. doi: 10.1007/BF00267078. [DOI] [PubMed] [Google Scholar]


