Abstract
Background
Pigment epithelium‐derived factor (PEDF), which belongs to the noninhibitory serpin family, has shown the ability to stimulate several physiological processes, such as antiangiogenesis, anti‐inflammation, and antioxidation. In the present study, the effects of PEDF on contractility and calcium handling of rat ventricular myocytes were investigated.
Methods and Results
Adult Sprague‐Dawley rat models of acute myocardial infarction (AMI) were surgically established. PEDF‐lentivirus was delivered into the myocardium along and away from the infarction border to overexpress PEDF. Video edge detection was used to measure myocyte shortening in vitro. Intracellular Ca2+ was measured in cells loaded with the Ca2+ sensitive fluorescent indicator, Fura‐2‐acetoxymethyl ester. PEDF local overexpression enhanced cardiac functional reserve in AMI rats and reduced myocardial contracture bordering the infracted area. Exogenous PEDF treatment (10 nmol/L) caused a significant decrease in amplitudes of isoproterenol‐stimulated myocyte shortening, Ca2+ transients, and caffeine‐evoked Ca2+ transients in vitro. We then tested a potential role for PEDF receptor‐mediated effects on upregulation of protein kinase C (PKC) and found evidence of signaling through the diacylglycerol/PKCα pathway. We also confirmed that pretreatment of cardiomyocytes with PEDF exhibited dephosphorylation of phospholamban at Ser16, which could be attenuated with PKC inhibition.
Conclusions
The results suggest that PEDF depresses myocyte contractility by suppressing phosphorylation of phospholamban and Ca2+ transients in a PKCα‐dependent manner through its receptor, PEDF receptor, therefore improving cardiac functional reserve during AMI.
Keywords: cardiomyocyte, contractility, myocardial infarction, pigment epithelial‐derived factor, pigment epithelial‐derived factor receptor
Subject Categories: Calcium Cycling/Excitation-Contraction Coupling, Contractile function, Ischemia, Myocardial Infarction
Introduction
Myocardial infarction (MI) is one of the leading causes of morbidity and mortality with death related to pump failure as well as to arrhythmias.1 In recent years, mortality with acute myocardial infarction (AMI) has decreased as efficient thrombolytic therapy and acute revascularization (angioplasty, stenting) have become available. However, preventing or reversing ventricular remodeling and the evolution toward end‐stage heart failure presents a new challenge.2 Various strategies are being explored, and the reduced contraction of the ischemic myocardium is considered to be 1 of the targets. Maintaining myocyte contractility post‐MI, by increasing Ca2+ influx, exacerbates cardiac injury and pump dysfunction post‐MI by reducing myocyte number.3 Therefore, the depressed myocyte contractility and Ca2+ influx may improve cardiac pump function and present a therapeutic strategy for AMI.
Calcium is the key factor of contractility. Development of contractility force depends on free intracellular Ca2+ concentration ([Ca2+]i) and total cytosolic [Ca2+] ([Ca2+]Tot) in highly nonlinear relations, as a result of strong myofilament cooperativity with respect to [Ca2+]i.4 There are 2 main ways to change the strength of cardiac contractility: by altering the amplitude of the Ca2+ transient and by altering the sensitivity of the myofilaments to Ca2+. The cardiac sarcoplasmic/endoplasmic reticulum Ca2+‐ATPase (SERCA2a) regulates intracellular Ca2+ handling and thus plays a critical role in initiating cardiac contraction and relaxation, and it could be modulated through its accessory phosphoprotein, phospholamban (PLN).5 Activation of protein kinase C alpha (PKCα) has been associated with protection from heart failure through augmented cardiac Ca2+ handling.6 The PKC family functions downstream of many signal transduction pathways,7 and PKCα is the predominant conventional PKC isoform expressed in the mouse, rat, and human heart.8 PKCα could be 1 of the pathways to decrease cardiac contractility through reducing sarcoplasmic reticulum (SR) Ca2+ load and Ca2+ release during systole through regulation of SERCA2a.
Pigment epithelium‐derived factor (PEDF) is a 50‐kDa secreted multifunctional protein of the SERPIN superfamily that has been implicated in many cardiovascular diseases.9, 10, 11 PEDF is expressed in multiple tissues and has many biological activities, such as antiangiogenesis, antioxidation, and cytoprotection.12, 13 PEDF could inhibit tissue remodeling and improve cardiac function through antioxidant and anti‐inflammatory actions in AMI rats.14 Our previous studies also found that PEDF could prevent hypoxia‐induced apoptosis and necroptosis in vitro and improve cardiac function post‐AMI in vivo.15, 16 However, whether PEDF affects cardiac contractility and myocyte calcium handling post‐AMI is still unclear.
PEDF mainly deposits in the cell membrane and interacts with its receptors. It is known that the 80‐kDa phospholipaseA2/nutrin/patarin‐like phospholipase domain‐containing 2 PEDF receptor (PEDF‐R) and 67‐kDa laminin receptor (LR) are two crucial receptors among PEDF binding sites.17, 18 PEDF‐R is the key enzyme of triglyceride (TG) catabolism and functions as a monoacyl hydrolase that catalyzes the initial, rate‐limiting step of the TG lipolysis cascade.19 PEDF‐R specifically initiates the first step in lipolysis, generating diacylglycerols (DAGs) and FAs. DAG accumulation can activate PKC by converting itself to phosphatidic acid through diacylglycerol kinase.20 Our previous studies have demonstrated that PEDF could promote TG degradation in cardiomyocytes post‐AMI through PEDF‐R.21 However, whether PEDF facilitates DAG accumulation and regulates PKCα signaling through PEDF‐R post‐AMI is still unknown.
In the present study, we hypothesized that PEDF upregulates DAG‐PKCα signaling and decreases intracellular calcium levels by PEDF‐R, thus depressing hypoxic myocyte contractility and improving ischemic cardiac functional reserve. This may provide a novel therapeutic target for AMI.
Methods
Recombinant Lentivirus Constructs and Viral Production
Recombinant PEDF‐lentivirus (PEDF‐LV) was prepared as described previously.16 PEDF overexpression plasmids and the RNAi vector, PEDF‐R‐RNAi‐LV, of the PEDF‐R gene producing PEDF‐R shRNA were successfully constructed and were then packaged in 293T cells. The RNAi vector, LR‐RNAi‐LV, of the LR gene was also constructed. The concentrated titer of virus suspension was 2×1012 TU/L.
Preparations of PEDF Protein
Recombinant rat PEDF (GenBankTM accession number: NM_177927) was synthesized by Cusabio Biotech, Co., Ltd. (Wuhan, China). In brief, 293T cells were transfected with the recombinant vector pGEX 6P‐1 glutathione S‐transferase (GST)‐tagged PEDF. GST‐tagged PEDF proteins were purified by high‐pressure liquid chromatography purification (>90% purity) and amino‐terminal sequence determination. The resulting proteins were soluble in aqueous solutions.
Animal Model and Intramyocardial Gene Delivery
Myocardial ischemia was induced by ligation of the left anterior descending coronary artery (LAD) in anesthetized rats, as described previously.22 Male Sprague‐Dawley rats (8–10 weeks old, weighing 200–250 g) were anesthetized with ketamine hydrochloride (INN ketamine) at 100 mg/kg and xylazine at 10 mg/kg, intubated with a 22‐gauge catheter, and artificially ventilated (Hallowell EMC, Pittsfield, MA). With the animal lying flat, left thoracotomy was performed through the fourth intercostal space, and the LAD was ligated with 6‐0 silk suture (Ethicon, Johnson & Johnson, Somerville, NJ) under direct vision. After ligation, PEDF‐LV (2×107 TU) in 20 μL of enhanced infection solution (ENIS; pH 7.4) was delivered with a 20‐μL syringe and 25‐gauge needle into the myocardium along and away the infarct border immediately. Control animals received an equivalent volume of LV vector in ENIS. The chest cavity was then closed, and animals were extubated and allowed to recover. Buprenorphine hydrochloride (INN buprenorphine) was administered at 0.5 mg/kg for postoperative analgesia. The animal models were randomly divided into 2 groups: group A (PEDF), PEDF‐LV was transferred after surgery; group B (vector control), LV vector was transferred into the ischemic myocardium. Animals were sacrificed with an overdose of sodium pentobarbitone (60 mg/kg, intravenously), and hearts were harvested at the end of 2 weeks after induction of MI for further analysis. All experimental protocols were approved by the animal care and use committee of Xuzhou Medical University (Xuzhou, China) and in compliance with the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication, 8th Edition, 2011).
Animal Cardiac Function Evaluation and Dobutamine Stress
Echocardiography was conducted under sedation by sodium pentobarbital (30 mg/kg, intraperitoneally), as described previously.23 It was performed at rest and during dobutamine stress at the end of 2 weeks after induction of MI. Two‐dimensional–guided M‐mode echocardiography was used to determine left ventricular chamber volume at systole and diastole and contractile parameters, such as left ventricular end‐diastolic dimension (LVEDD), left ventricular end‐systolic dimension (LVESD), left ventricular end‐diastolic volume (LVEDV), left ventricular end‐systolic volume (LVESV) and cardiac output (CO). Left ventricular fractional shortening (LVFS) was calculated as follows: FS (%)=(LVEDD−LVESD)/LVEDD×100. Ejection fraction (EF) was then derived as EF (%)=(EDV−ESV)/EDV×100. All measurements were based on the average of at least 3 cardiac cycles. Dobutamine (Sigma‐Aldrich, St. Louis, MO) 1 μg/g body weight was given intraperitoneally. Cardiac reserve was investigated 10 minutes after dobutamine injection.24
Quantification of Myocardial Infarct Size
Two weeks after LAD artery ligation, 2% Evans Blue dye (30 mg/kg; Sigma‐Aldrich) was injected intravenously for 10 minutes and selected rats were euthanized. Then, hearts were removed for myocardial infarct size analyses by the method of 2,3,5‐triphenyltetrazolium (TTC) staining.16 The left ventricle was isolated and cut into 4‐mm slices perpendicular to the axis of the LAD. Then, slices were immediately immersed in 1% TTC (Sigma‐Aldrich) in phosphate buffer (pH 7.4) at 37°C for 10 minutes to discriminate infarcted tissue from viable myocardium. All slices were scanned from both sides by a color CCD camera (FV‐10; Fujifilm, Tokyo, Japan), and in each slide, infarct area was compared with the total area by using digital planimetry software (Image‐Pro Plus 6.0; Media Cybernetics Inc., Bethesda, MD). After correction with the weight of the slices, infarct size was calculated as a percentage of the left ventricle.
Transmission Electron Microscopy Imaging and Analyses
For transmission electron microscopy observation, small samples of heart tissue were fixed in 2.5% glutaraldehyde overnight and then incubated in 1% osmium tetroxide for 2 hours with lightproof. After washing in distilled water, specimens were incubated in 2% uranyl acetate for 2 hours at room temperature and then dehydrated in graded ethanol concentrations. Finally, samples were embedded in molds with fresh resin. Ultrathin sections were cut with an EM UC7 (Leica Microsystems GmbH, Wetzlar, Germany), stained with lead citrate and examined with a Tecnai G2 T12 (FEI, Hillsboro, OR). In these images, we measured mean sarcomere length (SL) to test myocardial contracture by using digital planimetry software (Image‐Pro Plus 6.0; Media Cybernetics). Images with large SL heterogeneities were excluded from analysis.25
Rat Primary Cardiomyocytes Cultures and Hypoxia
Cardiomyocytes were isolated from 1‐day‐old newborn Sprague‐Dawley rats.26 In brief, neonatal rats were anesthetized with sodium pentobarbital and killed by means of decapitation, and hearts were rapidly removed and placed into dishes on ice. After atria and vessels were discarded, hearts were minced into 1‐mm3 pieces with sharp scissors, transferred to a sterile tube, and washed once in cold PBS solution (NaCl, 136.9 mmol/L; KCl, 2.7 mmol/L; Na2HPO4, 8.1 mmol/L; and KH2PO4, 1.5 mmol/L [pH 7.3]) to remove blood and clots. Minced tissue was digested in a PBS solution supplemented with 0.5% trypsin, 0.1% collagenase, and 0.02% glucose for 5 minutes at 37°C. The cell suspension was transferred into a tube containing 20 mL of DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco) and centrifuged at 200 g for 5 minutes. The cell pellet was resuspended in DMEM supplemented with 10% FBS and plated on 35‐mm dishes (Corning Incorporated, Corning, NY) at a density of 1.2×104/cm2 and cultured at 37°C in 5% CO2 and 95% air. Hypoxia was achieved by culturing cells in D‐Hank's liquid with glucose deprivation in a tri‐gas incubator (Heal Force, Shanghai, China) saturated with 5% CO2/1% O2 at 37°C for the indicated time periods.
Measurement of Single‐Cell Contractility
Myocytes were cultured for 24 hours in standard growth medium in Plexiglas superfusion chambers, in which the bottom was formed by a collagen‐coated glass coverslip. The chamber was then placed on the stage of an inverted microscope (Nikon Diaphot; Nikon Corporation, Tokyo, Japan), and cells were cultured in D‐Hank's liquid with glucose deprivation in a tri‐gas incubator saturated with 5% CO2/1% O2 at 37°C for 24 hours. Cell shortening was measured using a video edge detection system (Crescent Electronics, Sandy, UT). The signal was acquired using a Data Q DI‐200 board interfaced to a personal computer and stored using WinDaq software (DATAQ Instruments, Inc., Akron, OH). The cell image was also recorded on videotape for additional off‐line analysis. Amplitude of contraction under hypoxia condition was expressed relative to control values. Contractile amplitude under normoxia condition was set to 100%.
Measurement of Intracellular Ca2+ Concentration
Myocytes were loaded with the fluorescent indicator, Fura‐2‐acetoxymethyl ester (Fura 2‐AM; Molecular Probes, Eugene, OR) as described previously.27 In brief, 4 μL of a 1‐mmol/L stock solution of Fura 2‐AM (dissolved in DMSO) was added to 2 mL of cells to give a final Fura‐2 concentration of 2 μmol/L. Myocytes were shaken gently for 10 minutes at 24°C (room temperature). After loading, myocytes were centrifuged, washed with normal Tyrode (NT) to remove extracellular Fura‐2 and then placed for 15 minutes to ensure complete hydrolysis of the intracellular ester. To measure intracellular Ca2+ concentration, myocytes were alternately illuminated by 340‐ and 380‐nm light using an amonochromator (Cairn Research, Faversham, UK), which changed the excitation light every 2 ms. The resulting fluorescence emitted at 510 nm was recorded by a photomultiplier tube, and the ratio of the emitted fluorescence at the 2 excitation wavelengths (340/380 ratio) was calculated to provide an index of intracellular Ca2+ concentration. Caffeine (20 mmol/L) was then applied for 30 seconds using a customized rapid solution‐switching device. SR‐releasable Ca2+ was assessed by measuring the area under the curve of the caffeine‐evoked Ca2+ transient.
ELISA Analysis
Myocytes were seeded into 96‐well plates (1×104 cells/well) and cultured for 3 days. After the respective treatments, cardiac cells were washed with NT and added with PMSF. Then, the cell lysis buffer was harvested and spun at 800g for 5 minutes with supernatant filtered (0.45 μm). Samples were transferred to antibody‐coated plates. The concentration of DAG was determined by competitive inhibition ELISA (USCN, Wuhan, China). Plate preparation and assay procedure were performed according to the manufacturer's recommendations. Absorbance was read in a reference wavelength of 450 nm. DAG concentration for each sample was calculated after generating a standard curve by a microplate reader (BioTek Synergy2; BioTek Instruments, Inc., Winooski, VT).
Western Blotting Analysis
For western blotting analysis, cells were solubilized in lysis buffer (100 mmol/L of Tris‐HCl, 4% SDS, 20% glycerine, 200 mmol/L of dithiothreitol, and protease inhibitors [pH 6.8]). Total cellular protein was denatured by boiling for 10 minutes with an equal volume of 23 Tris‐glycine SDS buffer. Protein was separated by 10% SDS‐PAGE and transferred to nitrocellulose membrane (Millipore, Billerica, MA). After blocking with 5% nonfat milk/PBS‐T for 3 hours at room temperature, membranes were incubated with antibodies, including PKCα (Bioworld Technology, Inc., St Louis Park, MN), PLN (Thermo Fisher Scientific, Waltham, MA), phosphorylated PLN (pPLN; Thermo Fisher Scientific), SERCA2a (Sigma‐Aldrich), and β‐tublin (Bioworld Technology). Then, fluorescently labeled secondary antibody (Rockland, Inc., Limerick, PA) was added for 1 hour and subsequently scanned by the Odyssey Infrared Imaging System (LI‐COR Biosciences, Lincoln, NE).
Statistical Analysis
Results are expressed as the means±SE. Statistical analysis of the results was carried out using the Student t test or repeated‐measures 1‐way ANOVA followed by Duncan's new multiple range method or least significant difference test. P<0.05 was considered significant.
Results
PEDF Increases Cardiac Functional Reserve and Reduces Ischemic Contracture in AMI Rat Hearts
Of the 14 rats entered into the present experiment, they were divided into PEDF or vehicle treatment groups. We first examined expression of PEDF and PEDF‐R after 2 weeks of MI (Figure 1A). Results showed that protein levels of PEDF decreased and protein levels of PEDF‐R increased 2 weeks post‐AMI (Figure 1B and 1C). Then, the PEDF‐LV was successfully transfected into myocardium and confirmed by its green fluorescent protein (GFP) fluorescence (Figure 1D). ELISA analysis showed that protein levels of PEDF in the PEDF‐transfected group significantly increased compared to those of the control group and could reach up to 9.55 nmol/L (Figure 1E). Cardiac contractile function was measured at 2 weeks post‐MI using transthoracic M‐mode echocardiography before and after dobutamine (1 μg/g) injection (Figure 2A and 2B). The delta ejection fraction (ΔEF) and delta fractional shortening to dobutamine infusions were both significantly higher in hearts transfected with PEDF compared to untreated MI hearts (Figure 2C and 2D). Evaluation of cardiac reserve (stress‐rest) clearly indicated that PEDF treatment had increased delta CO, compared to the MI group, during dobutamine challenge (Figure 2E). In addition, PEDF treatment also showed reduced myocardial infarct size compared to the vector control in AMI rats (Figure 2F and 2G).
Figure 1.
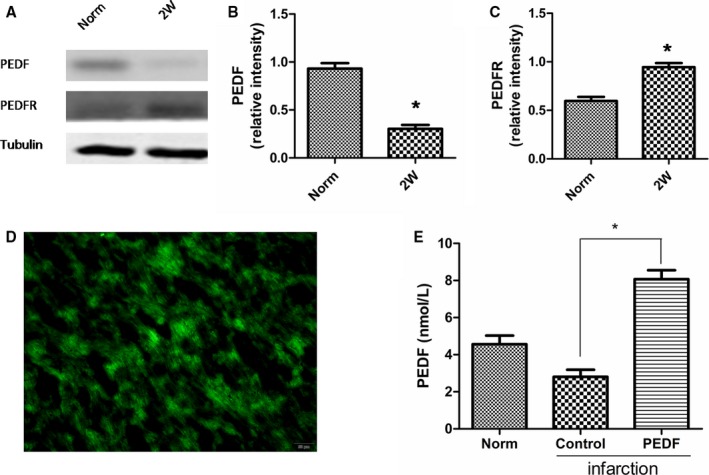
Expression of pigment epithelium‐derived factor (PEDF) and PEDF receptor (PEDF‐R) after acute myocardial infarction (AMI) and the validity of PEDF transfection. ELISA analysis was used to test PEDF content. The plasmid lentivirus was constructed and labeled with green fluorescent protein. A through C, Expression of PEDF and PEDF‐R post‐AMI. Data are shown as mean±SE from 3 samples (n=3). *P<0.001 versus respective normal (norm) rats. Results show that the protein levels of PEDF decreased and the protein levels of PEDF‐R increased 2 weeks post‐AMI. D, Transfection of PEDF in lentivirus‐injected myocardium. E, Quantification of PEDF content in lentivirus‐injected myocardium 2 weeks post‐AMI. Data are shown as mean±SE from 6 samples (n=6). *P<0.001. Results show that the protein levels of PEDF in the PEDF‐transfected group significantly increased compared to those of the control group.
Figure 2.
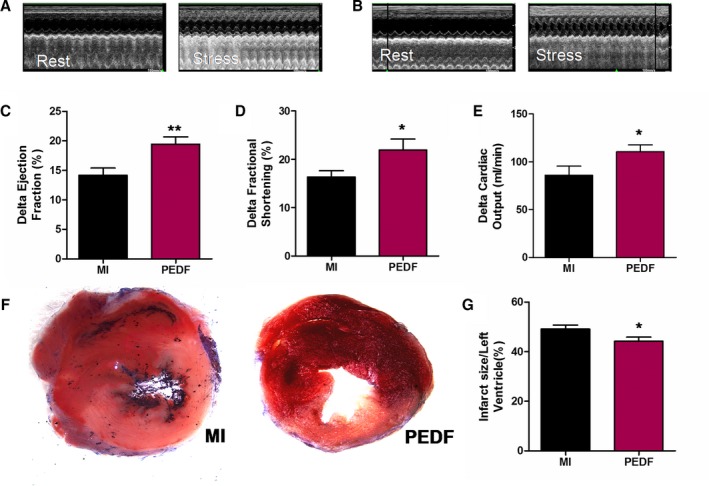
Evaluation of cardiac functional reserve after acute myocardial infarction (AMI). Cardiac function was measured in pigment epithelium‐derived factor (PEDF)‐transfected myocardial infarction (MI) hearts using transthoracic M‐mode echocardiography. Measurements were performed in rats before injection of dobutamine (1 μg/g) and 10 minutes after injection at 2 weeks post‐AMI. The 2 groups included vector transfected rats (MI) and PEDF‐transfected rats (PEDF). A, Representative echocardiograms of an MI rat before and after dobutamine injection. B, Representative echocardiograms of a PEDF‐transfected rat before and after dobutamine injection. C, Delta left ventricular ejection fraction. D, Delta left ventricular fractional shortening. E, Delta left ventricular cardiac output. F, Representative images of 2,3,5‐triphenyltetrazolium‐stained myocardial tissues. G, Quantification of infarct size. Data are shown as mean±SE from 7 hearts (n=7). *P<0.05 versus MI; **P<0.01 versus MI. Results show that PEDF significantly improved cardiac functional reserve and reduced myocardial infarct size when compared to the MI group.
We next examined myocardial tissues transfected with PEDF or vector at cellular levels. Cross‐sections with an ellipticity larger than 0.96 were not used for further analysis. Figure 3A through 3D shows representative images from each group. Interestingly, the bordering areas transfected with PEDF have a longer SL than the areas in the MI group (Figure 3F). However, we did not find any significant difference in remote areas between the PEDF and MI groups (Figure 3E). Electronic microscope observations reveal that PEDF may protect bordering myocardial tissues from ischemic contracture.
Figure 3.
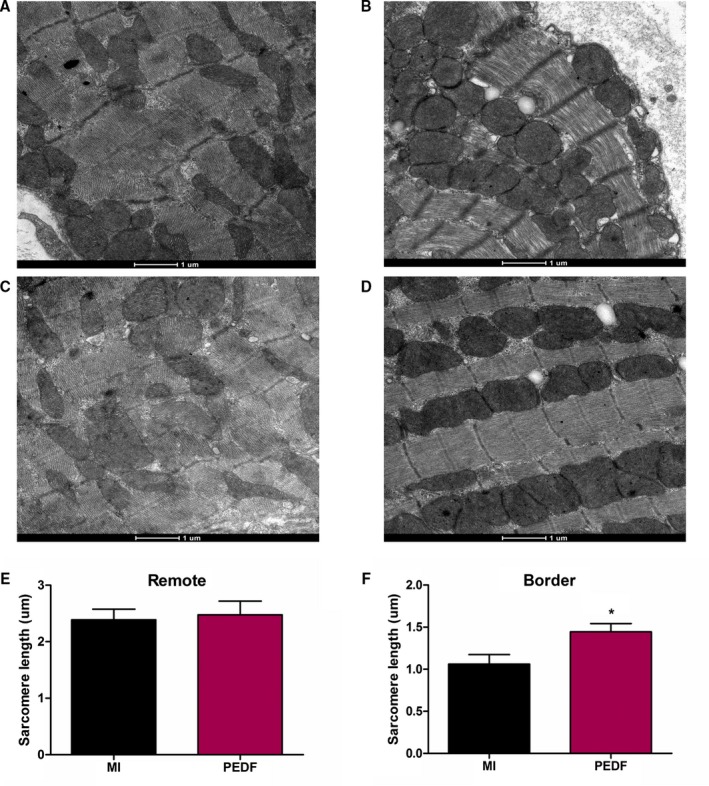
Myocardial contracture in the infracted heart 2 weeks after myocardial infarction (MI). A, Representative image of MI remote area. B, Representative image of MI border area. C, Representative image of pigment epithelium‐derived factor (PEDF) remote area. D, Representative image of PEDF border area. E, Sarcomere length in remote area. F, Sarcomere length in border area. Data are shown as mean±SE from 6 hearts (n=6). **P<0.05 versus MI. Results show an increased sarcomere length in the peri‐infarct region of the PEDF‐transfected group when compared to the MI group. No significant difference was observed in the remote areas between PEDF and MI rats. Results show that PEDF protected myocardial tissues from ischemic contracture.
PEDF Promotes a Rapid Depression of Contractility in Hypoxic Neonatal Cardiomyocytes
Cultured rat neonatal cardiomyocytes provide a useful model to understand cardiovascular diseases in vitro on a real‐time cell analysis system, so we further verified the protective effects of PEDF on contractility in neonatal cardiomyocytes. The RNAi vector, PEDF‐R‐RNAi‐LV, of the PEDF‐R gene producing PEDF‐R shRNA and LR were successfully transfected in neonatal cardiomyocytes and were then successfully confirmed by its GFP fluorescence (Figure 4D). Immunostaining for α‐SA showed the establishment of neonatal cardiomyocytes in vitro (Figure 4A through 4C). A video edge detection system was used to monitor the effects of PEDF (10 nmol) on hypoxia‐induced single‐cell contraction changes. Spontaneously beating myocytes added with isoproterenol (1 μm) were exposed to hypoxia for 24 hours with or without PEDF pretreatment. Similarly, PEDF had no significant effect on cell contractility in cultured neonatal cardiomyocytes under normal culture conditions (Figure 5A and 5C). Contractility was then measured during the exposure to hypoxia period (Figure 5B and 5D). All groups’ contractions decreased with the extension of hypoxic time and PEDF pretreatment, showing a more‐rapid time‐dependent trend. PEDF caused a significant decrease in contraction amplitude of spontaneously beating myocytes after 3 hours of hypoxia that persisted throughout the following 8 hours until it was stable. These results clearly demonstrated that PEDF promotes a rapid and significant decrease of contractility in hypoxic neonatal cardiomyocytes, but has no effects on normoxic cardiomyocytes.
Figure 4.
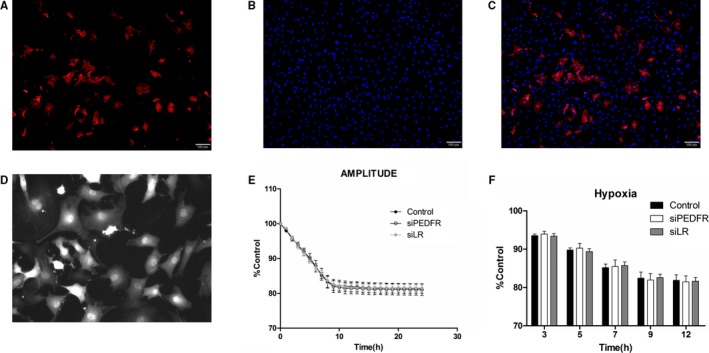
Establishment of neonatal cardiomyocytes and tracking of contraction changes in neonatal cardiomyocytes. Cardiomyocytes with or without PEDF‐R‐RNAi‐LV or LR‐RNAi‐LV pretransfected were cultured for 24 hours under hypoxia condition. The plasmid lentivirus was constructed and labeled with green fluorescent protein. A video edge detection system was used to monitor single‐cell contraction changes. The 3 groups included hypoxia (control), PEDF‐R‐RNAi‐LV under hypoxia (siPEDF‐R) and LR‐RNAi‐LV under hypoxia (siLR). A through C, Fluorescent images of 1 of the cultures labeled with antibodies against cardiac α‐actin. D, Tramsfection of siPEDF‐R and siLR in neonatal cardiomyocytes. E and F, Amplitude of contraction under hypoxia condition was detected without PEDF treatment. Data are shown as mean±SE from 6 cardiomyocytes (n=6). No significant difference was observed among PEDF, siPEDF‐R, and siLR groups. Data are expressed relative to control values measured at the start of each experiment. LV indicates lentivirus; PEDF, pigment epithelium‐derived factor; PEDF‐R, PEDF receptor.
Figure 5.
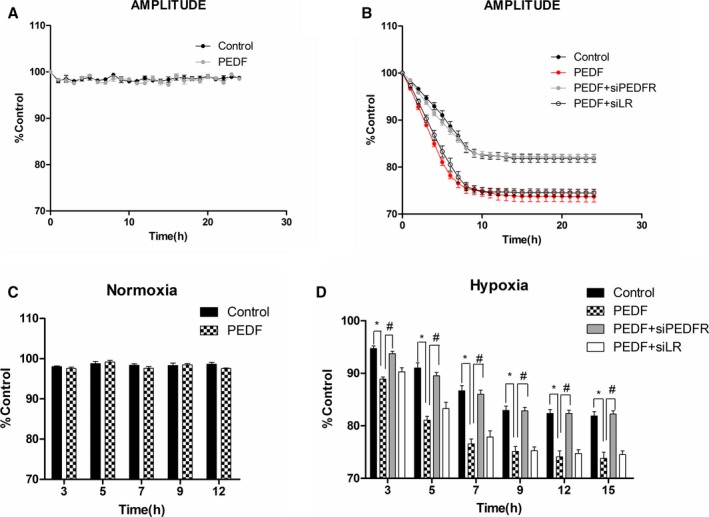
Tracking of contraction changes in neonatal cardiomyocytes. Cardiomyocytes with or without PEDF‐R‐RNAi‐LV or LR‐RNAi‐LV pretransfected were cultured for 24 hours under hypoxia condition. A video edge detection system was used to monitor single‐cell contraction changes. The 4 groups included hypoxia (control), PEDF under hypoxia (PEDF), PEDF with PEDF‐R‐RNAi‐LV under hypoxia (PEDF+siPEDF‐R), and PEDF with LR‐RNAi‐LV under hypoxia (PEDF+siLR). A and C, Amplitude of contraction under normoxia condition. Data are shown as mean±SE from 6 cardiomyocytes (n=6). No significant difference was observed between PEDF and control groups. B, Amplitude of contraction under hypoxia condition. Data are shown as mean±SE from 8 cardiomyocytes (n=8). P<0.001 PEDF versus control; P<0.001 PEDF+siPEDF‐R versus PEDF. D, Statistical analysis of several time points under hypoxia condition. Data are shown as mean±SE from 8 cardiomyocytes (n=8). *P<0.01; # P<0.01. Data are expressed relative to control values measured at the start of each experiment. Results show that PEDF (10 nmol)‐pretreated cardiomyocytes showed a more‐rapid time‐dependent contraction reduction, and the effects could be attenuated by PEDF‐R interference. No significant difference was observed between PEDF and PEDF+siLR groups. LV indicates lentivirus; PEDF, pigment epithelium‐derived factor; PEDF‐R, PEDF receptor.
PEDF Decreases Intracellular Calcium Levels in Hypoxic Neonatal Cardiomyocytes
Curves of [Ca2+]i levels were measured in beating cardiomyocytes isolated from neonatal cardiomyocytes, which were driven by isoproterenol stimulation (Figure 6A through 6F). Consistent with depressed cellular contractility, hypoxic neonatal myocytes with PEDF treatment (10 nmol) showed decreased Ca2+ transient amplitudes compared to the hypoxia group (Figure 6H). Similarly, PEDF also had no influence on normoxia cardiomyocytes. We next investigated the SR Ca2+ load after 12 hours of hypoxia in cultured cardiomyocytes (Figure 7A). We confirmed less SR Ca2+ loads in PEDF‐treated cardiomyocytes compared to hypoxia groups (Figure 7B). Furthermore, PEDF treatment (10 nmol) cardiomyocytes showed reduced PLN phosphorylation, which would decrease SERCA2 activity and SR Ca2+ load (Figure 7C and 7D). No other changes were observed in levels of PLN and SERCA2 proteins in cultured neonatal cardiomyocytes. Together, these results suggest that PEDF decreases intracellular calcium levels through PLN dephosphorylation in hypoxic neonatal cardiomyocytes.
Figure 6.
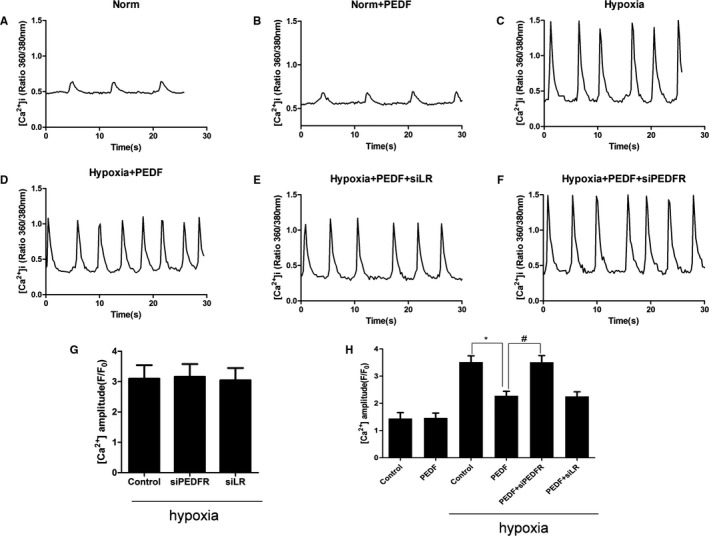
Calcium influx in neonatal cardiomyocytes. Curves of [Ca2+]i level were measured in beating cardiomyocytes after 12 hours of hypoxia, which were driven by isoproterenol (1 μmol/L) stimulation. The 6 groups included normal (Norm), PEDF under normal (Norm+PEDF), hypoxia (Hypoxia), PEDF under hypoxia (Hypoxia+PEDF), PEDF with LR‐RNAi‐LV under hypoxia (Hypoxia+PEDF+siLR), and PEDF with PEDF‐R‐RNAi‐LV under hypoxia (Hypoxia+PEDF+siPEDF‐R). A through F, Curves of calcium influx in hypoxic cardiomyocytes. G, Calcium influx in hypoxic cardiomyocytes. No significant difference was observed among these 3 groups. Data are shown as mean±SE from 6 cardiomyocytes (n=6). H, Isoproterenol stimulation induced Ca2+ transients from these groups measured as a change in fluorescence (F/F0). Data are shown as mean±SE from 6 cardiomyocytes (n=6). *P<0.05; # P<0.01. Results show that PEDF decreased Ca2+ transient amplitudes when compared to control group under hypoxia condition and PEDF‐R interference could attenuate the effect. LV indicates lentivirus; PEDF, pigment epithelium‐derived factor; PEDF‐R, PEDF receptor.
Figure 7.
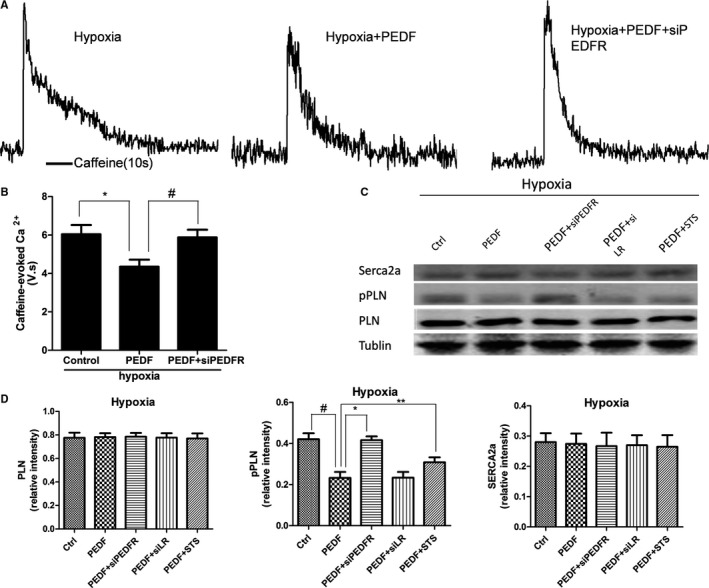
Sarcoplasmic reticulum (SR) Ca2+ load and proteins expression in neonatal cardiomyocytes. Another group added as PEDF (10 nmol) with staurosporine (STS) under hypoxia (PEDF+STS). A, Representative caffeine‐induced Ca2+ transients from Hypoxia, Hypoxia+PEDF, and Hypoxia+PEDF+siPEDF‐R neonatal cardiomyocytes. B, Quantification of the area under caffeine‐evoked Ca2+ transients in rat ventricular myocytes. Data are shown as mean±SE from 6 cardiomyocytes (n=6). *P<0.05; # P<0.05. Results show that PEDF reduced sarcoplasmic reticulum (SR) Ca2+ load and PEDF‐R interference also could attenuate the effect. C and D, Sarcoplasmic/endoplasmic reticulum Ca2+‐ATPase (Serca2a), phosphoylated phospholamban (pPLN), and phospholamban (PLN) expression in hypoxic cardiomyocytes. Data are shown as mean±SE from 3 samples (n=3). *P<0.01; # P<0.01; **P<0.05. Results show that PEDF reduced PLN phosphorylation under hypoxia condition through PEDF‐R and the protein kinase C alpha inhibitor, staurosporine, could attenuate the effects of PLN dephosphorylation. No significant difference was observed in Serca2a and PLN. PEDF indicates pigment epithelium‐derived factor; PEDF‐R, PEDF receptor.
PEDF Decreases Hypoxic Cardiomyocyte Contractility and Intracellular Calcium Levels Through PEDF‐R
To investigate which receptor was involved in PEDF effects on contractility depression and intracellular calcium decrease, the PEDF‐R‐siRNA (siRNA)‐lentivirus (PEDF‐R‐RNAi‐LV) and LR‐RNAi‐LV were administered. We first confirmed that neither PEDF‐R‐LV nor LR‐LV alone changed the contractions (Figure 4E and 4F) and intracellular calcium (Figure 6G) of hypoxic cardiomyocytes. PEDF‐treated (10‐nmol) cultures plus PEDF‐R‐LV caused a significant increase in contraction amplitude of spontaneously beating cardiomyocytes compared with PEDF alone under hypoxia condition (Figure 5B and 5D). A significant increase in intracellular calcium levels was also observed in PEDF‐treated cultures plus PEDF‐R‐LV compared to the PEDF group (Figures 6H and 7B). PEDF treatment myocytes plus PEDF‐R‐LV also showed increased PLN phosphorylation (Figure 7D). Nevertheless, there was no significant difference between PEDF‐treatment cardiomyocytes and PEDF plus LR‐LV under hypoxia condition. These results indicated that the effects of PEDF on contractility and calcium handling of hypoxic cardiomyocytes are mediated through PEDF‐R.
PEDF Decreases Hypoxic Cardiomyocyte Contractility and Intracellular Calcium Levels Through DAG‐PKCα Signaling
The PKCα pathway inhibitor was utilized in these experiments. We first discovered that PEDF (10 nmol) upregulates protein expression of PKCα through PEDF‐R signaling (Figure 8B). Notably, PEDF did not increase protein levels of PKCα under normal condition. The PKCα inhibitor, staurosporine (STS), plus PEDF was able to attenuate the effects of PLN dephosphorylation in hypoxic cardiomyocytes (Figure 7C and 7D). No other changes were observed in levels of PLN and SERCA2 proteins in cultured neonatal myocytes. Next, we tested whether PEDF upregulates the protein, PKCα, in a DAG‐dependent manner. ELISA analysis showed that PEDF induced diglyceride increase in hypoxic cardiomyocytes (Figure 8A). The increase was also inhibited by PEDF‐R‐RNAi‐LV, and there were no changes under normal condition. These results indicated that PEDF promotes DAG accumulation and upregulates PKCα through PEDF‐R. PEDF may decrease hypoxic cardiomyocyte contractility and intracellular calcium levels through DAG‐PKCα signaling with PEDF‐R involved in the process.
Figure 8.
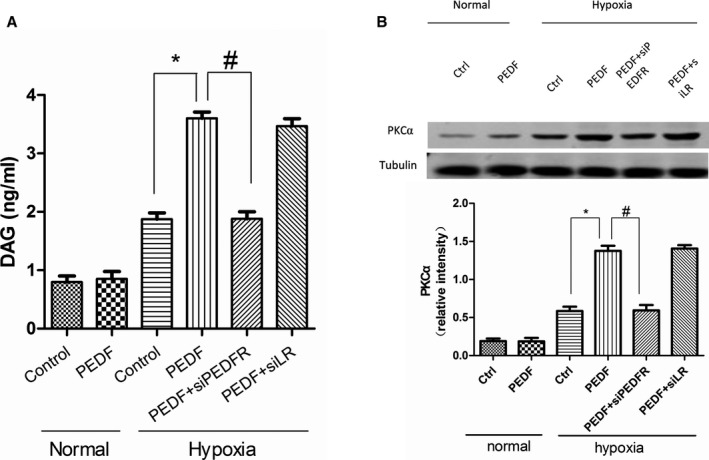
Diacylglycerol (DAG) content and protein kinase C alpha (PKCα) regulation in neonatal cardiomyocytes. ELISA analysis were used to test DAG content. A, Quantification of DAG content in cardiomyocytes after 12 hours of hypoxia. Data are shown as mean±SE from 4 samples (n=4). *P<0.001; # P<0.001. Results show that PEDF (10 nmol) induced diglyceride increase in hypoxic cardiomyocytes through PEDF‐R and had no effects under normal condition. B, PKCα protein expression in cardiomyocytes. Data are shown as mean±SE from 3 samples (n=3). *P<0.01; # P<0.01. Results show that PKCα significantly increased in the PEDF group compared to the control group in hypoxic cardiomyocytes. PEDF indicates pigment epithelium‐derived factor; PEDF‐R, PEDF receptor.
Discussion
The results of this study indicate, for the first time, that PEDF depresses hypoxic myocyte contractility and improves ischemic cardiac functional reserve through its receptor, PEDF‐R, and DAG‐PKCα signaling and intracellular calcium participate in the effects of PEDF on hypoxic myocyte contractility and ischemic cardiac functional reserve.
In our experiments, we used video edge detection system to measure single‐cell‐level contractility in a relatively isolated environment without the influence of autonomic nerve endings, gap junctions, and a neurotransmitter uptake system. We found that PEDF caused a significant reduction in the hypoxic myocyte contraction and calcium amplitudes without altering them under a normal oxygen environment. These findings provide evidence that the negative inotropic effect of PEDF just exists under hypoxic condition. In combination with the electronic microscope observations, PEDF probably decreases the myocardial contraction in bordering infarction areas and has no effects on remote regions. This may also explain the difference of contractile changes in vitro and in vivo. The fascinating difference may be attributed to the cardiomyocyte contraction changes in the bordering zones of MI. The reduced contraction of ischemic myocardial is considered to be an adaptive response with contraction perfusion matching.28 PEDF may decrease the ischemic cardiomyocyte contractility in bordering areas and thus reduce the energy expenditure to adapt to the anoxic environment. Therefore, this response reduces energy consumption and ischemic myocardial contracture and brings better cardiac functional reserve post‐AMI.
PEDF was originally identified as a secreted protein found in high levels in circulation.29 The binding of PEDF to its receptor is presumably the first step in the mediation of its physiological effects. PEDF signaling is triggered by binding of PEDF to its receptors, PEDF‐R and LR. In our experiments, we have demonstrated that PEDF promotes a significant decrease in contractility in hypoxic neonatal cardiomyocytes through its receptor, PEDF‐R, but has no effect on normoxic cells. This discrepancy above may be attributed to the increased expression of PEDF‐R in hypoxic cells. LR was not involved in the process of contraction. Unlike LR involved in the antiangiogenic activity of endothelial cells,18 PEDF‐R may mediate diverse physiological effects in cardiomyocytes. PEDF may perform different biological functions in different tissues through different receptors.
Meanwhile, some investigations about the effect of PEDF on cardiomyocytes are still controversial. Our in vitro study showed that PEDF could prevent hypoxia‐induced apoptosis and necroptosis in cardiomyocytes, whereas Rychli et al. have shown that circulating PEDF may contribute to progression of heart failure by inducing apoptosis in human cardiac myocytes and fibroblasts.30 Given that PEDF is constitutively expressed by cardiac myocytes and fibroblasts, the increase of PEDF plasma concentration may be attributed to the release of injured cells. Under anoxic conditions, PEDF expression was reduced both in cardiomyocytes and ischemic myocardium. We therefore used PEDF LVs and exogenous proteins to upregulate local PEDF expression and found that PEDF decreases ischemic myocardial contracture and improves functional reserve in vivo and reduces hypoxic cardiomyocyte contractility in vitro. PEDF is especially needed for its beneficial effects under hypoxic conditions.
Our previous in vitro studies have established that 10 nmol/L is the optimal concentration of myocardial protection. So, we used the concentration in our experiments. We also injected LVs into ischemic cardiac tissues to continuously overexpress PEDF and confirmed that the protein levels of PEDF in vivo could sufficiently reach the requirement of the optimal concentration in vitro.
Our present study is the first to report the participation of DAG‐PKCα signaling and intracellular calcium in PEDF signaling. We found that PEDF induced DAG accumulation and increased expression of PKCα in hypoxic cardiomyocytes. PKCα has been associated with regulating myocytes calcium handling and contractility.6 Thus, PEDF may decrease hypoxic cardiomyocyte calcium handling and contractility through DAG‐PKCα signaling through PEDF‐R. DAG accumulation may be associated with PEDF‐R property, which catalyzes the initial, rate‐limiting step of the TG lipolysis cascade.19 Specifically, DAG has been shown in a variety of model systems to activate PKC family kinases and mediates diverse physical activities.31, 32 Our finding of DAG accumulation may broaden the potential clinical value of PEDF for its hydrolysis property. To establish the essential role of PKCα in PEDF signaling, we used PKCα antagonist to inhibit PKCα and confirmed the participation of PKCα in dephosphorylation of PLN.
One final issue is how PEDF mechanistically reduces hypoxic cardiomyocyte contractility. Alterations in PLN phosphorylation regulate SERCA2a function in the heart, which controls calcium loading and magnitude of the calcium transient.5 Here, we observed a similar reduction in PLN phosphorylation at serine16 in PEDF‐treated cardiomyocytes. Thus, PEDF represents an approach toward achieving hypoxic contractility depression through an SR‐dependent mechanism, which could benefit MI. Alternatively, it is also possible that PEDF reduces cardiac contractility through some mechanisms involving the phosphorylation status of select myofilament proteins or other unknown signaling. Regardless of these possibilities, our present data strongly suggest that PEDF decreases intracellular calcium levels through DAG‐PKCα signaling through PEDF‐R, therefore depressing hypoxic myocyte contractility and improving ischemic cardiac functional reserve.
Sources of Funding
This work was supported by the National Nature Science Foundation of China (81570242) and the Technology Bureau of Xuzhou of China (Grant No. KC14SH106).
Disclosures
None.
Acknowledgments
The authors thank Yan Yan (Department of Medical Imaging, Affiliated Hospital of Xuzhou Medical University) for the echocardiography testing.
(J Am Heart Assoc. 2016;5:e003179 doi: 10.1161/JAHA.115.003179)
References
- 1. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray J, Pfeffer MA. New therapeutic options in congestive heart failure: part I. Circulation. 2002;105:2099–2106. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Chen X, Gao E, MacDonnell SM, Wang W, Kolpakov M, Nakayama H, Zhang X, Jaleel N, Harris DM, Li Y, Tang M, Berretta R, Leri A, Kajstura J, Sabri A, Koch WJ, Molkentin JD, Houser SR. Increasing cardiac contractility after myocardial infarction exacerbates cardiac injury and pump dysfunction. Circ Res. 2010;107:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bers DM. Cardiac excitation‐contraction coupling. Nature. 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 5. Periasamy M, Huke S. Serca pump level is a critical determinant of Ca(2+) homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001;33:1053–1063. [DOI] [PubMed] [Google Scholar]
- 6. Liu Q, Chen X, Macdonnell SM, Kranias EG, Lorenz JN, Leitges M, Houser SR, Molkentin JD. Protein kinase Cα, but not PKCβ or PKCγ, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly‐Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. [DOI] [PubMed] [Google Scholar]
- 8. D'Souza KM, Petrashevskaya NN, Merrill WH, Akhter SA. Inhibition of protein kinase C alpha improves myocardial beta‐adrenergic receptor signaling and ventricular function in a model of myocardial preservation. J Thorac Cardiovasc Surg. 2008;135:172–179, 179.e171. [DOI] [PubMed] [Google Scholar]
- 9. Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi‐functional serpin family protein. J Cell Biochem. 2009;106:769–775. [DOI] [PubMed] [Google Scholar]
- 10. Mahtabifard A, Merritt RE, Yamada RE, Crystal RG, Korst RJ. In vivo gene transfer of pigment epithelium‐derived factor inhibits tumor growth in syngeneic murine models of thoracic malignancies. J Thorac Cardiovasc Surg. 2003;126:28–38. [DOI] [PubMed] [Google Scholar]
- 11. Rychli K, Kaun C, Hohensinner PJ, Dorfner AJ, Pfaffenberger S, Niessner A, Bauer M, Dietl W, Podesser BK, Maurer G, Huber K, Wojta J. The anti‐angiogenic factor PEDF is present in the human heart and is regulated by anoxia in cardiac myocytes and fibroblasts. J Cell Mol Med. 2010;14:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium‐derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. [DOI] [PubMed] [Google Scholar]
- 13. Truong A, Wong TY, Khachigian LM. Emerging therapeutic approaches in the management of retinal angiogenesis and edema. J Mol Med (Berl). 2011;89:343–361. [DOI] [PubMed] [Google Scholar]
- 14. Ueda S, Yamagishi S, Matsui T, Jinnouchi Y, Imaizumi T. Administration of pigment epithelium‐derived factor inhibits left ventricular remodeling and improves cardiac function in rats with acute myocardial infarction. Am J Pathol. 2011;178:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao X, Zhang H, Zhuang W, Yuan G, Sun T, Jiang X, Zhou Z, Yuan H, Zhang Z, Dong H. PEDF and PEDF‐derived peptide 44mer protect cardiomyocytes against hypoxia‐induced apoptosis and necroptosis via anti‐oxidative effect. Sci Rep. 2014;4:5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H, Wang Z, Feng SJ, Xu L, Shi HX, Chen LL, Yuan GD, Yan W, Zhuang W, Zhang YQ, Zhang ZM, Dong HY. PEDF improves cardiac function in rats with acute myocardial infarction via inhibiting vascular permeability and cardiomyocyte apoptosis. Int J Mol Sci. 2015;16:5618–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Notari L, Baladron V, Aroca‐Aguilar JD, Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML, Sanchez‐Sanchez F, Escribano J, Laborda J, Becerra SP. Identification of a lipase‐linked cell membrane receptor for pigment epithelium‐derived factor. J Biol Chem. 2006;281:38022–38037. [DOI] [PubMed] [Google Scholar]
- 18. Bernard A, Gao‐Li J, Franco CA, Bouceba T, Huet A, Li Z. Laminin receptor involvement in the anti‐angiogenic activity of pigment epithelium‐derived factor. J Biol Chem. 2009;284:10480–10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner‐Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. [DOI] [PubMed] [Google Scholar]
- 20. Takeda M, Kagaya Y, Takahashi J, Sugie T, Ohta J, Watanabe J, Shirato K, Kondo H, Goto K. Gene expression and in situ localization of diacylglycerol kinase isozymes in normal and infarcted rat hearts: effects of captopril treatment. Circ Res. 2001;89:265–272. [DOI] [PubMed] [Google Scholar]
- 21. Zhang H, Sun T, Jiang X, Yu H, Wang M, Wei T, Cui H, Zhuang W, Liu Z, Zhang Z, Dong H. PEDF and PEDF‐derived peptide 44mer stimulate cardiac triglyceride degradation via ATGL. J Transl Med. 2015;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, Qin Y, Zhang P, Chen Y, Harada M, Isse T, Kawamoto T, Fan H, Yang P, Akazawa H, Nagai T, Takano H, Ping P, Komuro I, Ge J. Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc. 2014;3:e000779 doi: 10.1161/JAHA.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta2‐adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev. 2005;10:289–296. [DOI] [PubMed] [Google Scholar]
- 24. Dodic M, Samuel C, Moritz K, Wintour EM, Morgan J, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89:623–629. [DOI] [PubMed] [Google Scholar]
- 25. McNary TG, Spitzer KW, Holloway H, Bridge JH, Kohl P, Sachse FB. Mechanical modulation of the transverse tubular system of ventricular cardiomyocytes. Prog Biophys Mol Biol. 2012;110:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun M, Ouzounian M, de Couto G, Chen M, Yan R, Fukuoka M, Li G, Moon M, Liu Y, Gramolini A, Wells GJ, Liu PP. Cathepsin‐l ameliorates cardiac hypertrophy through activation of the autophagy‐lysosomal dependent protein processing pathways. J Am Heart Assoc. 2013;2:e000191 doi: 10.1161/JAHA.113.000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howarth FC, Qureshi MA. Effects of carbenoxolone on heart rhythm, contractility and intracellular calcium in streptozotocin‐induced diabetic rat. Mol Cell Biochem. 2006;289:21–29. [DOI] [PubMed] [Google Scholar]
- 28. Bito V, Heinzel FR, Weidemann F, Dommke C, van der Velden J, Verbeken E, Claus P, Bijnens B, De Scheerder I, Stienen GJ, Sutherland GR, Sipido KR. Cellular mechanisms of contractile dysfunction in hibernating myocardium. Circ Res. 2004;94:794–801. [DOI] [PubMed] [Google Scholar]
- 29. Petersen SV, Valnickova Z, Enghild JJ. Pigment‐epithelium‐derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. Biochem J. 2003;374:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rychli K, Niessner A, Hohensinner PJ, Mahdy Ali K, Kaun C, Neuhold S, Zorn G, Richter B, Hulsmann M, Berger R, Mortl D, Huber K, Maurer G, Pacher R, Wojta J. Prognostic value of pigment epithelium‐derived factor in patients with advanced heart failure. Chest. 2010;138:656–664. [DOI] [PubMed] [Google Scholar]
- 31. Liu YM, Wang X, Nawaz A, Kong ZH, Hong Y, Wang CH, Zhang JJ. Wogonin ameliorates lipotoxicity‐induced apoptosis of cultured vascular smooth muscle cells via interfering with DAG‐PKC pathway. Acta Pharmacol Sin. 2011;32:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Ma K, Sun P, Liu S, Qin H, Zhu Z, Wang X, Yan Q. LeY oligosaccharide upregulates DAG/PKC signaling pathway in the human endometrial cells. Mol Cell Biochem. 2009;331:1–7. [DOI] [PubMed] [Google Scholar]


